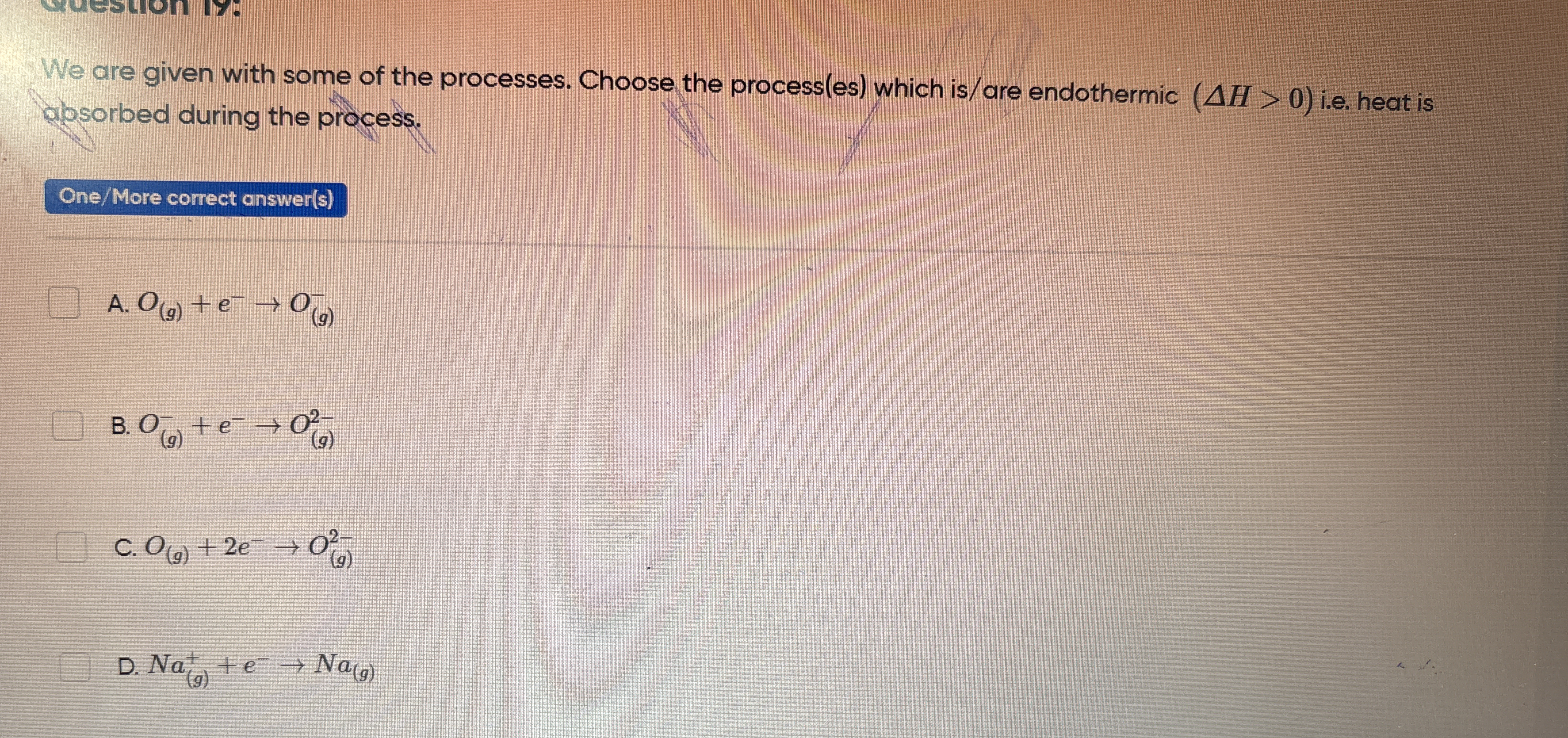
We are given with some of the processes. Choose the process(es) which is/are endothermic (ΔH > 0) i.e. heat is absorbed during the process.
Understand the Problem
The question is asking to identify which of the given chemical processes are endothermic, meaning they absorb heat (ΔH > 0). This involves assessing each provided reaction to determine if heat is absorbed.
Answer
C and D are endothermic.
The endothermic processes are C and D.
Answer for screen readers
The endothermic processes are C and D.
More Information
In the given processes, C involves adding more electrons to neutral atoms, which often requires energy. Similarly, D involves converting Na⁺ to Na, a process that typically requires energy input due to the electrostatic attraction that must be overcome.
Tips
A common mistake is to confuse exothermic and endothermic processes. Remember that endothermic processes absorb heat, resulting in a positive ΔH.
Sources
- Endothermic vs. exothermic reactions (article) - Khan Academy - khanacademy.org
- Endothermic and exothermic processes (video) - Khan Academy - khanacademy.org
AI-generated content may contain errors. Please verify critical information