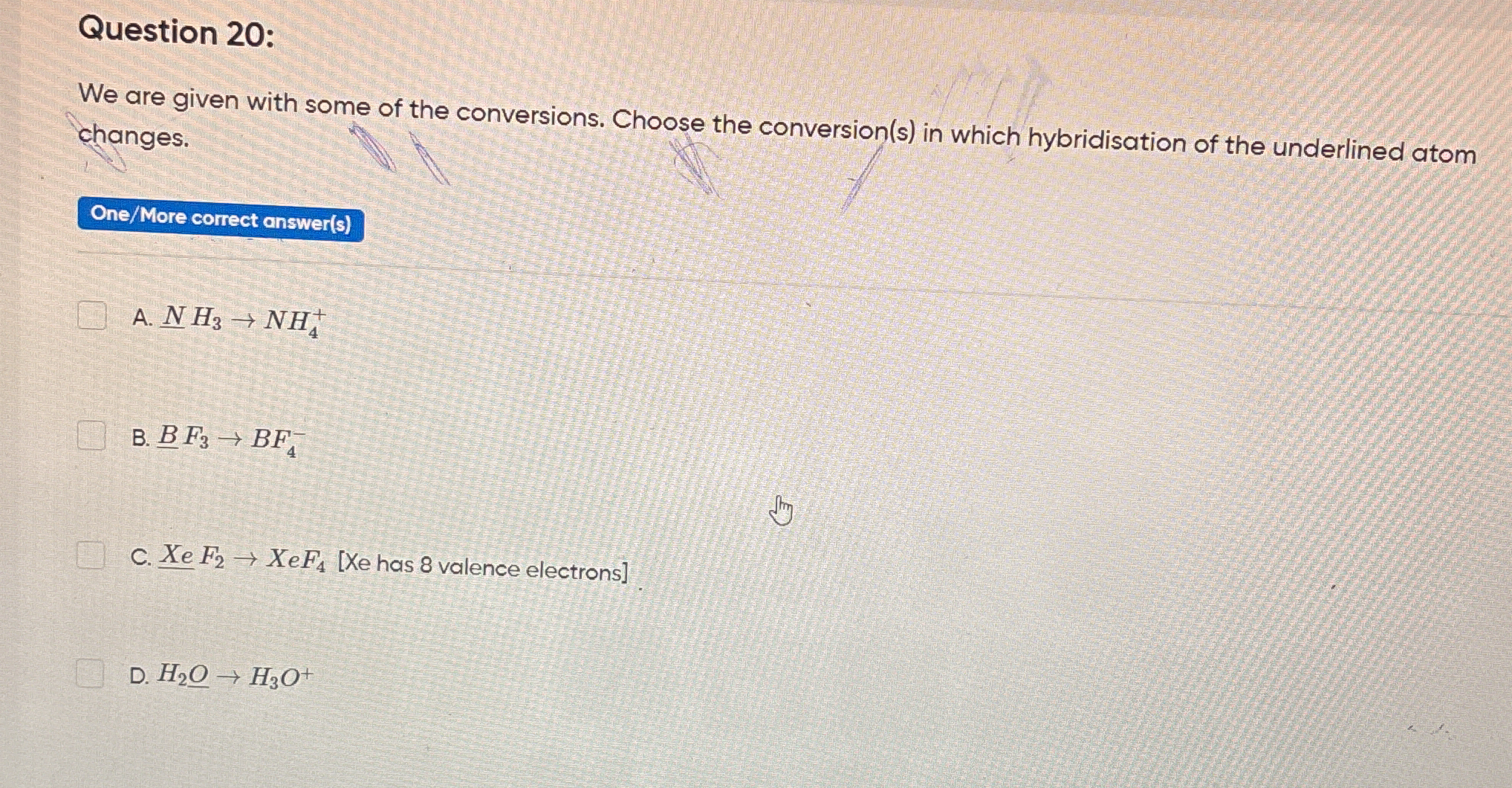
We are given some of the conversions. Choose the conversion(s) in which hybridisation of the underlined atom changes. A. NH3 → NH4+ B. BF3 → BF4- C. XeF2 → XeF4 [Xe has 8 valence e... We are given some of the conversions. Choose the conversion(s) in which hybridisation of the underlined atom changes. A. NH3 → NH4+ B. BF3 → BF4- C. XeF2 → XeF4 [Xe has 8 valence electrons] D. H2O → H3O+
Understand the Problem
The question asks which conversions among the given options lead to a change in the hybridization of the underlined atom in each conversion. This involves understanding hybridization concepts in chemistry.
Answer
B and C
The hybridization changes in conversions B and C.
Answer for screen readers
The hybridization changes in conversions B and C.
More Information
In the BF3 to BF4- conversion, boron adds an extra electron pair, resulting in a transition from sp2 to sp3 hybridization. Similarly, in the conversion from XeF2 to XeF4, xenon uses more orbitals, going from sp3d to sp3d2 hybridization.
Tips
A common mistake is overlooking the need to consider the addition of electron pairs, which can change hybridization.
Sources
AI-generated content may contain errors. Please verify critical information