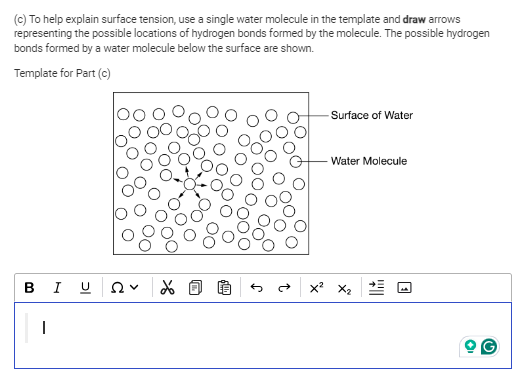
To help explain surface tension, use a single water molecule in the template and draw arrows representing the possible locations of hydrogen bonds formed by the molecule.
Understand the Problem
The question is asking to illustrate the location of hydrogen bonds formed by a water molecule at the surface of water, using a template provided where one water molecule is highlighted. The task involves drawing arrows to show the potential hydrogen bonding interactions.
Answer
Draw arrows from the oxygen atom to adjacent water molecules, showing up to four hydrogen bonds.
To represent hydrogen bonds, draw arrows from the oxygen atom towards adjacent water molecules. A water molecule can form up to four hydrogen bonds – with its own hydrogens and those of neighboring molecules.
Answer for screen readers
To represent hydrogen bonds, draw arrows from the oxygen atom towards adjacent water molecules. A water molecule can form up to four hydrogen bonds – with its own hydrogens and those of neighboring molecules.
More Information
In water, hydrogen bonds occur due to the attraction between the slightly positive hydrogen atoms of one molecule and the slightly negative oxygen atom of another. These bonds are responsible for water's high surface tension.
Tips
A common mistake is not showing all four potential hydrogen bonds or incorrectly placing the arrows. Ensure arrows indicate interaction with neighboring molecules.
Sources
- Hydrogen Bonds Make Water Sticky - University of Hawaii at Manoa - manoa.hawaii.edu
AI-generated content may contain errors. Please verify critical information