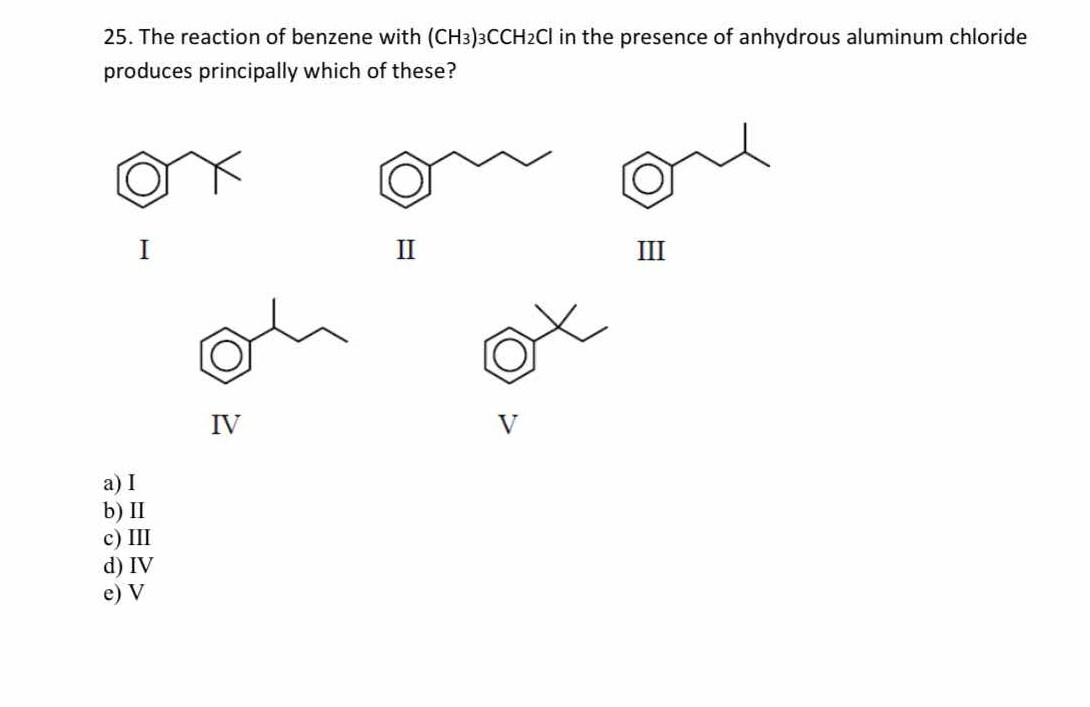
The reaction of benzene with (CH3)3CCH2Cl in the presence of anhydrous aluminum chloride produces principally which of these?
Understand the Problem
The question is asking which compound is primarily produced when benzene reacts with isopropyl chloride in the presence of anhydrous aluminum chloride. This involves understanding the electrophilic aromatic substitution reaction and the products formed.
Answer
V
The final answer is V.
Answer for screen readers
The final answer is V.
More Information
The reaction is a Friedel-Crafts alkylation, where the (CH3)3CCH2Cl provides a t-butyl carbocation. This carbocation rearranges to form a more stable carbocation, which then adds to the benzene ring, resulting in the t-butylbenzene product.
Tips
Be careful about carbocation rearrangements, as more stable carbocations will form.
Sources
- Brainly question on the reaction - brainly.com
AI-generated content may contain errors. Please verify critical information