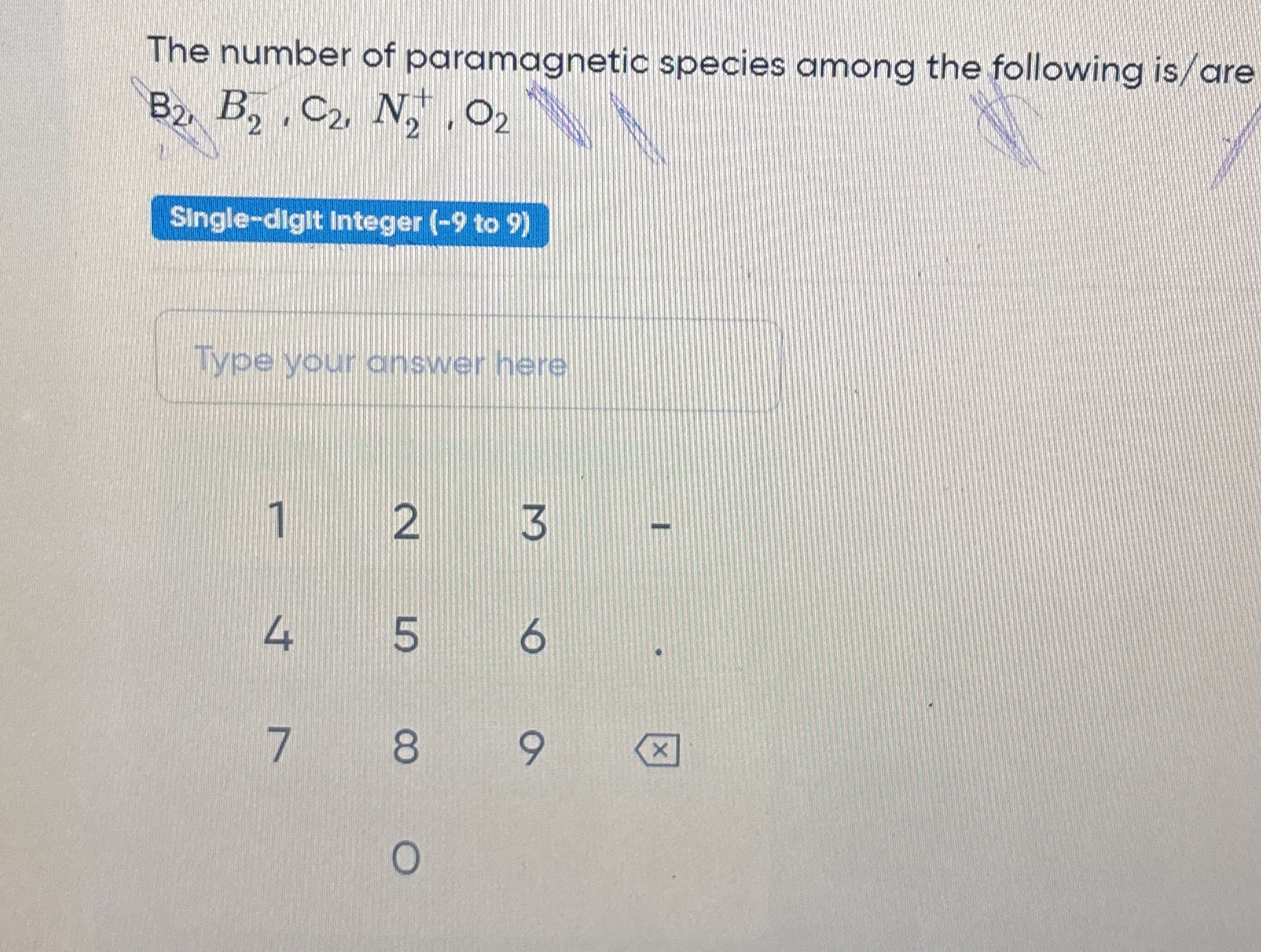
The number of paramagnetic species among the following is/are B2, C2, N+, O2?
Understand the Problem
The question is asking for the count of paramagnetic species among the given chemical species, which includes B2, C2, N+, and O2. To solve it, we need to determine the number of unpaired electrons in each species.
Answer
2
The paramagnetic species are B2 and O2.
Answer for screen readers
The paramagnetic species are B2 and O2.
More Information
Molecular orbital theory shows that B2 has two unpaired electrons and O2 has two unpaired electrons in π* orbitals, making both paramagnetic.
Tips
A common mistake is overlooking the molecular orbital diagram to correctly identify unpaired electrons.
Sources
- The number of paramagnetic species among the following is ______ - infinitylearn.com
AI-generated content may contain errors. Please verify critical information