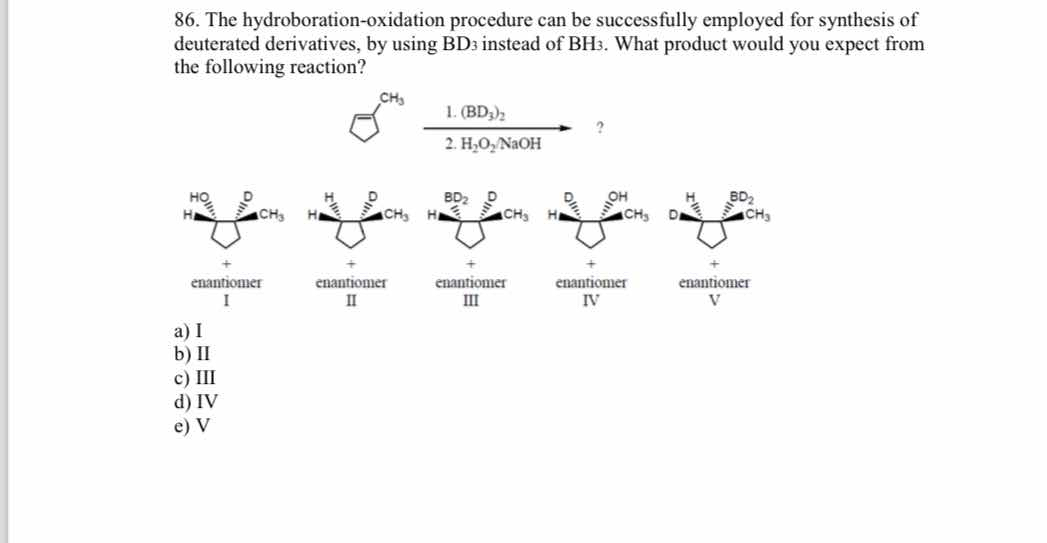
The hydroboration-oxidation procedure can be successfully employed for synthesis of deuterated derivatives, by using BD3 instead of BH3. What product would you expect from the foll... The hydroboration-oxidation procedure can be successfully employed for synthesis of deuterated derivatives, by using BD3 instead of BH3. What product would you expect from the following reaction?
Understand the Problem
The question involves a chemical reaction, specifically the hydroboration-oxidation process using BD3 to produce a deuterated derivative. The user is asked to identify the expected product from the reaction described, along with several options for products presented as enantiomers.
Answer
IV
The product of the reaction is enantiomer IV.
Answer for screen readers
The product of the reaction is enantiomer IV.
More Information
Hydroboration-oxidation proceeds with syn addition of BH3 (or BD3), leading to an alcohol with anti-Markovnikov regioselectivity. Using BD3 replaces BH3, incorporating deuterium instead.
Tips
Ensure recognition of syn addition and anti-Markovnikov regioselectivity when predicting products.
Sources
- Hydroboration-Oxidation Reaction Mechanism - Master Organic Chemistry - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information