Understand the Problem
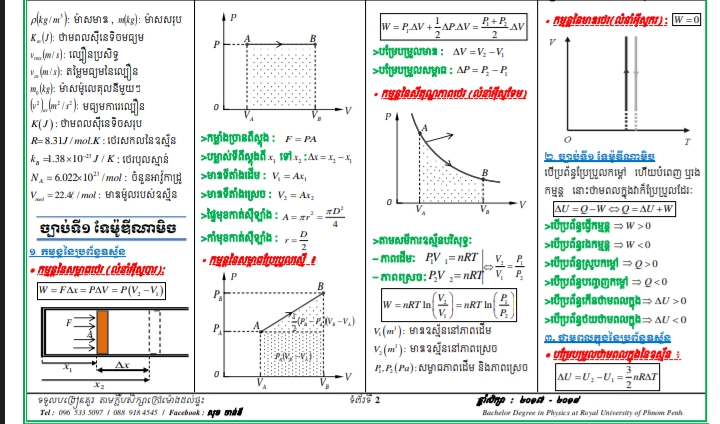
The question is asking about the concepts and calculations related to work done during pressure and volume changes in a gas system, as evidenced by the equations and diagrams presented.
Answer
Interpreting thermodynamic principles using PV diagrams and key equations.
The final answer involves interpreting thermodynamic principles using PV diagrams and key equations like the ideal gas law and work done by/on a gas.
Answer for screen readers
The final answer involves interpreting thermodynamic principles using PV diagrams and key equations like the ideal gas law and work done by/on a gas.
More Information
This image encompasses various thermodynamic processes such as work done by gases, changes in internal energy, and relationships explained by the ideal gas law. It visually and textually represents these concepts for educational purposes.
Tips
A common mistake students make is confusing the direction of work done by or on the gas. Work done by the gas during expansion is considered positive, while work done on the gas during compression is considered negative.
AI-generated content may contain errors. Please verify critical information