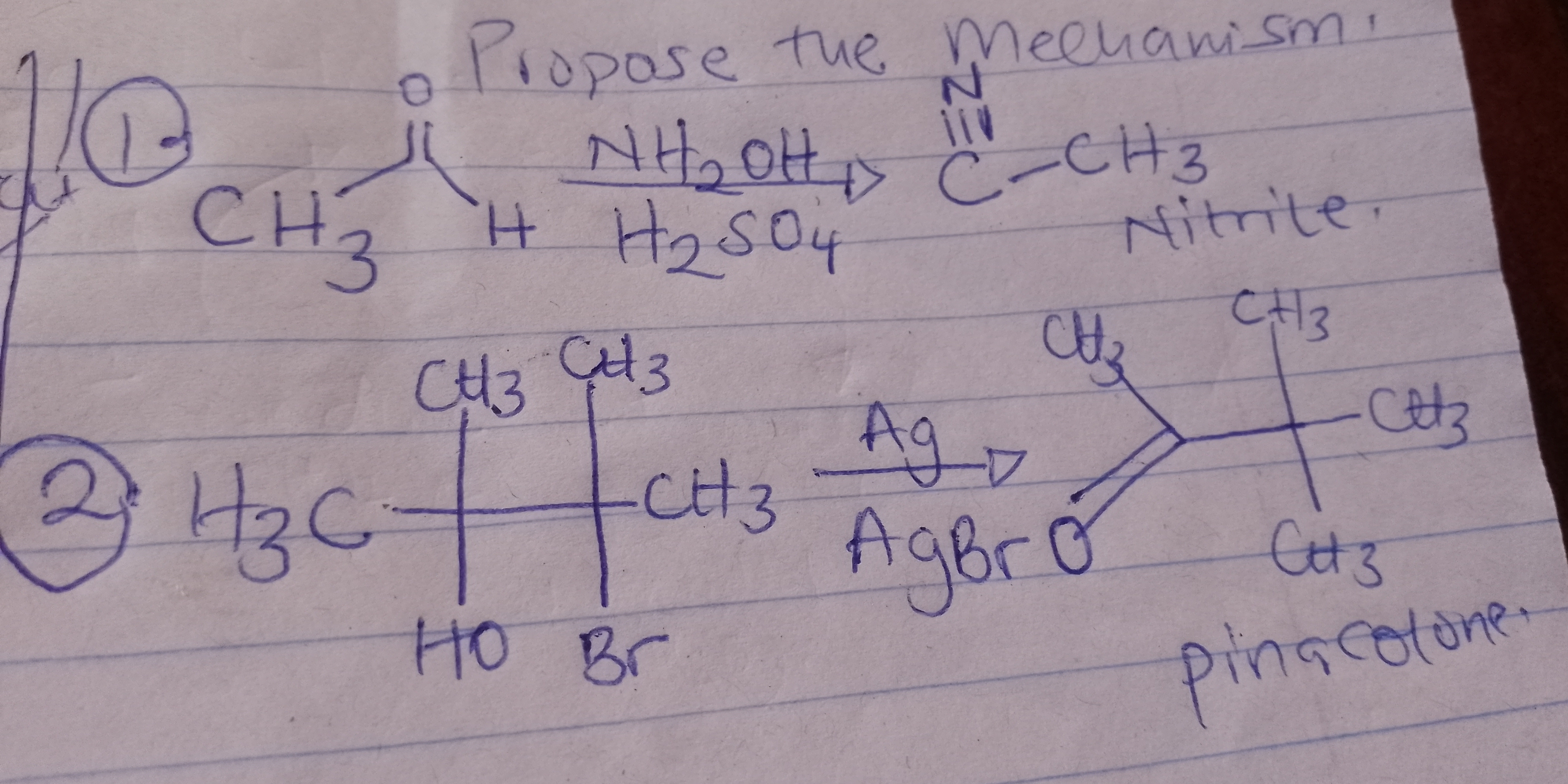
Propose the mechanism for the following reactions: 1. Nitrile reaction with CH3 and H2SO4. 2. Ag reaction with CH3 and Br producing pinacolone.
Understand the Problem
The question is asking for the mechanisms involved in two organic reactions, one involving the conversion of a nitrile and the other involving a reaction that produces pinacolone. This indicates a need to outline detailed steps or processes that occur in these chemical reactions.
Answer
Nitrile to carboxylic acid, methyletion; Ag assists Br removal for rearrangement to pinacolone.
In the first reaction, the nitrile is hydrolyzed to a carboxylic acid, then methylated. In the second, Ag+ removes Br, forming an intermediate that rearranges to pinacolone.
Answer for screen readers
In the first reaction, the nitrile is hydrolyzed to a carboxylic acid, then methylated. In the second, Ag+ removes Br, forming an intermediate that rearranges to pinacolone.
More Information
In the first reaction, nitriles undergo hydrolysis in acidic conditions to first form amides, then acids. Methylation occurs after forming the acid. In the second, Ag+ ions help eliminate bromide, causing a rearrangement typical of a pinacol-pinacolone transformation.
Tips
Ensure correct identification of intermediates and conditions (acidic for hydrolization, presence of silver for halide removal).
Sources
AI-generated content may contain errors. Please verify critical information