Understand the Problem
The question is likely related to the concepts of anodes and cathodes in electrochemistry, explaining their functions and characteristics in an electrolytic cell.
Answer
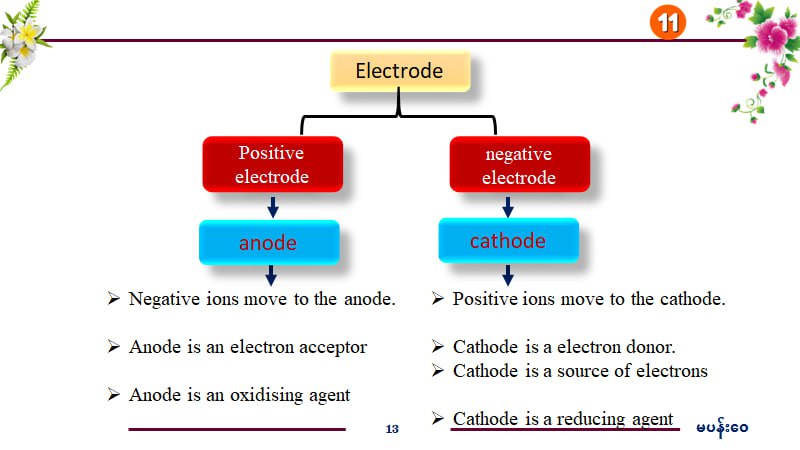
Anode is a positive electrode; cathode is negative.
The anode is the positive electrode where negative ions move, it acts as an electron acceptor and is an oxidizing agent. The cathode is the negative electrode where positive ions move, acts as an electron donor, and is a reducing agent.
Answer for screen readers
The anode is the positive electrode where negative ions move, it acts as an electron acceptor and is an oxidizing agent. The cathode is the negative electrode where positive ions move, acts as an electron donor, and is a reducing agent.
More Information
The anode attracts negative ions and the cathode attracts positive ions in electrochemical cells.
Tips
A common mistake is confusing the roles of anodes and cathodes in different types of cells.
AI-generated content may contain errors. Please verify critical information