ગણનાનો આધાર M2 ધરાવતી પ્રવાહી W2 માટે દીલકશન કરવાનો આશય શું છે?
Understand the Problem
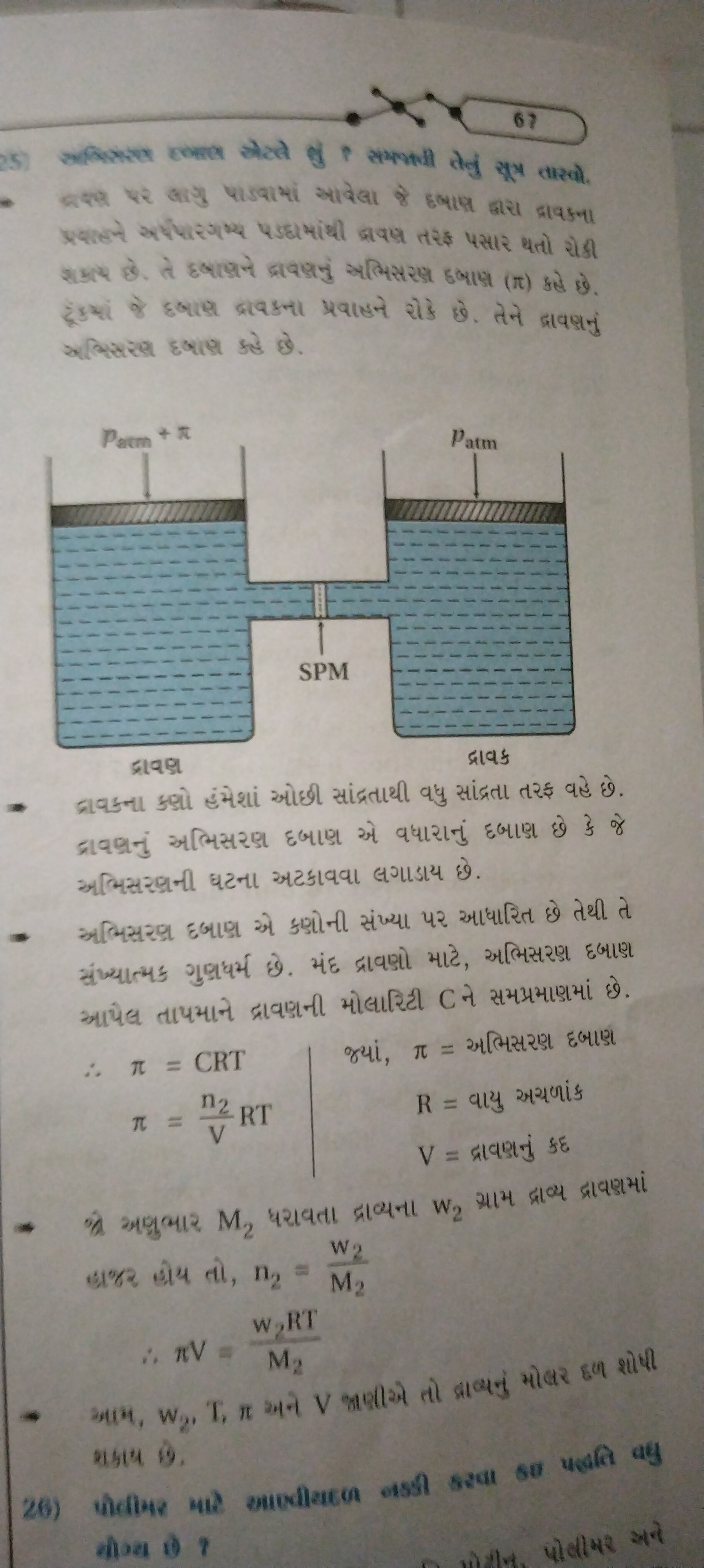
પ્રશ્નમાં જે ચાર્ટ અને તારવી સામગ્રી રજૂ છે તે પ્રવાહશીલતા અને તાપમાન સાથેના સંબં તેમણે સમજીને ગણના કરવાની જરૂર છે.
Answer
The derived relationship is $\pi V = \frac{w_2 RT}{M_2}$.
Answer for screen readers
The relationship derived from the formulas will yield $\pi V = \frac{w_2 RT}{M_2}$.
Steps to Solve
-
Understand the context of the problem The problem discusses fluid flow and its relationship with pressure and temperature using given formulas.
-
Identify the formulas used The following formulas are provided:
- The ideal gas law: $\pi = CRT$
- For different conditions of gas in the two chambers:
- $\pi = \frac{n_2}{V}RT$
- The relationship between the parameters: $\pi V = \frac{w_2 RT}{M_2}$
-
Define variables
- Let $w_2$ be the weight of the gas, $M_2$ be its molar mass, $R$ be the universal gas constant, and $T$ be the temperature.
- Initial values and conditions might need to be established for specific calculations.
-
Rearranging the equation
- Using the relationship $\pi V = \frac{w_2 RT}{M_2}$ to solve for $\pi$ or $V$ depending on the requirement.
-
Calculate the values
- Plug in the known values of $w_2$, $T$, and $M_2$ into the rearranged formula to find $\pi V$.
-
Interpret results
- Analyze how changes in temperature, pressure, or weight of the gas could affect the overall behavior in the system.
The relationship derived from the formulas will yield $\pi V = \frac{w_2 RT}{M_2}$.
More Information
This relates to the application of the ideal gas law in practical scenarios, illustrating how temperature and pressure interrelate with the properties of a gas in various states.
Tips
- Forgetting to convert units: Ensure all inputs are in compatible units (e.g., pressure in atm, volume in liters).
- Misapplying the ideal gas law: Each scenario may have specific conditions or constants that differ; use appropriate values.