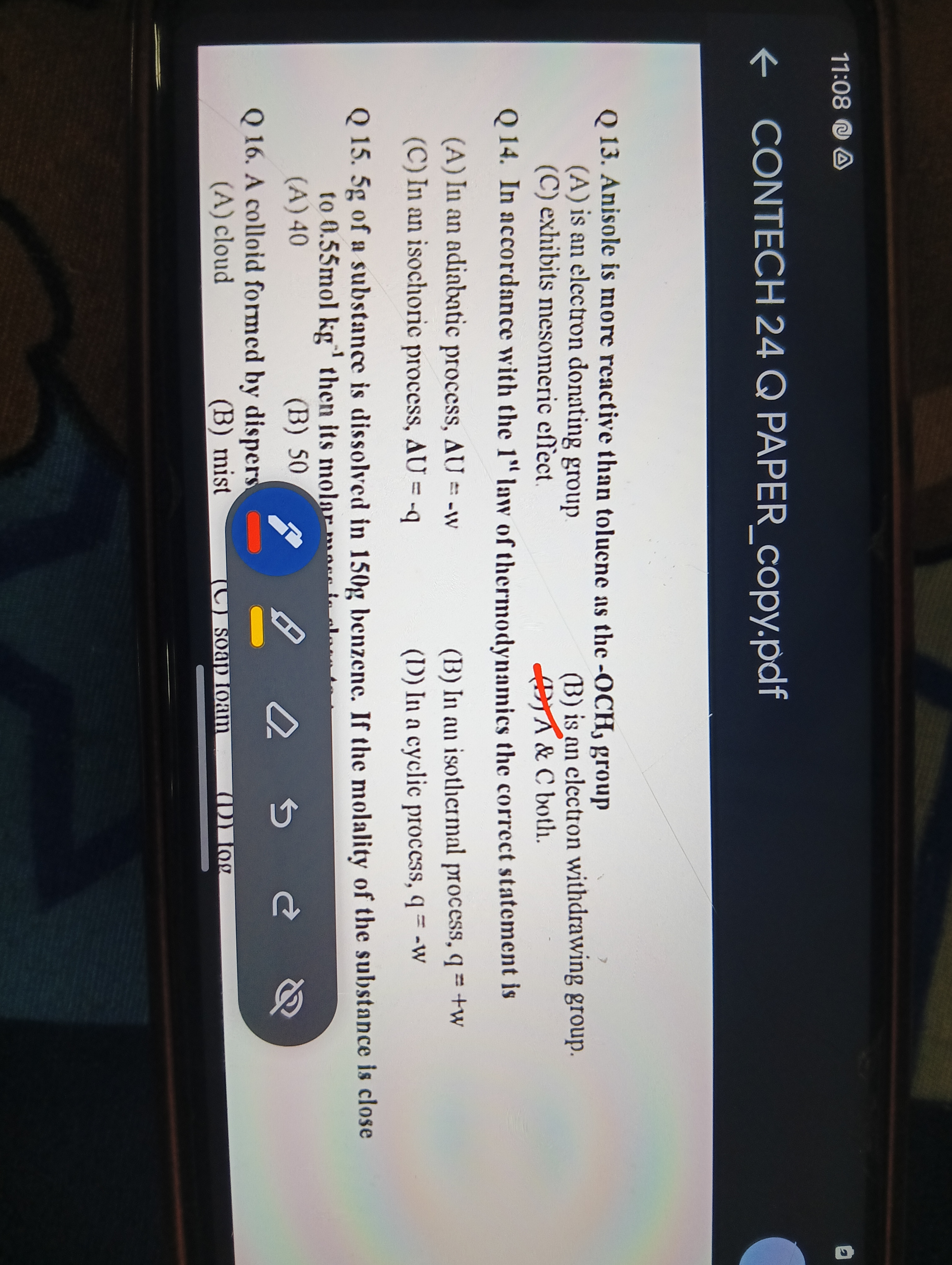
In accordance with the 1st law of thermodynamics, the correct statement is: (A) In an adiabatic process, delta U = -w (B) In an isochoric process, delta U = -q (C) In an isothermal... In accordance with the 1st law of thermodynamics, the correct statement is: (A) In an adiabatic process, delta U = -w (B) In an isochoric process, delta U = -q (C) In an isothermal process, q = w (D) In a cyclic process, q = w.
Understand the Problem
The question is asking which statement is correct regarding thermodynamic processes, specifically whether certain relationships and definitions hold true in those contexts.
Answer
(C) q = w in isothermal; (D) q = w in cyclic process.
The correct statements are (C) In an isothermal process, q = w, and (D) In a cyclic process, q = w.
Answer for screen readers
The correct statements are (C) In an isothermal process, q = w, and (D) In a cyclic process, q = w.
More Information
In an isothermal process, the internal energy change (ΔU) is zero, thus q = w. In a cyclic process, the net change in internal energy is also zero, so the heat absorbed equals the work done, q = w.
Tips
Ensure you understand the characteristics of specific processes like adiabatic, isochoric, isothermal, and cyclic when applying the first law.
Sources
- The First Law of Thermodynamics - Chemistry LibreTexts - chem.libretexts.org
- Adiabatic process - Wikipedia - en.wikipedia.org
AI-generated content may contain errors. Please verify critical information