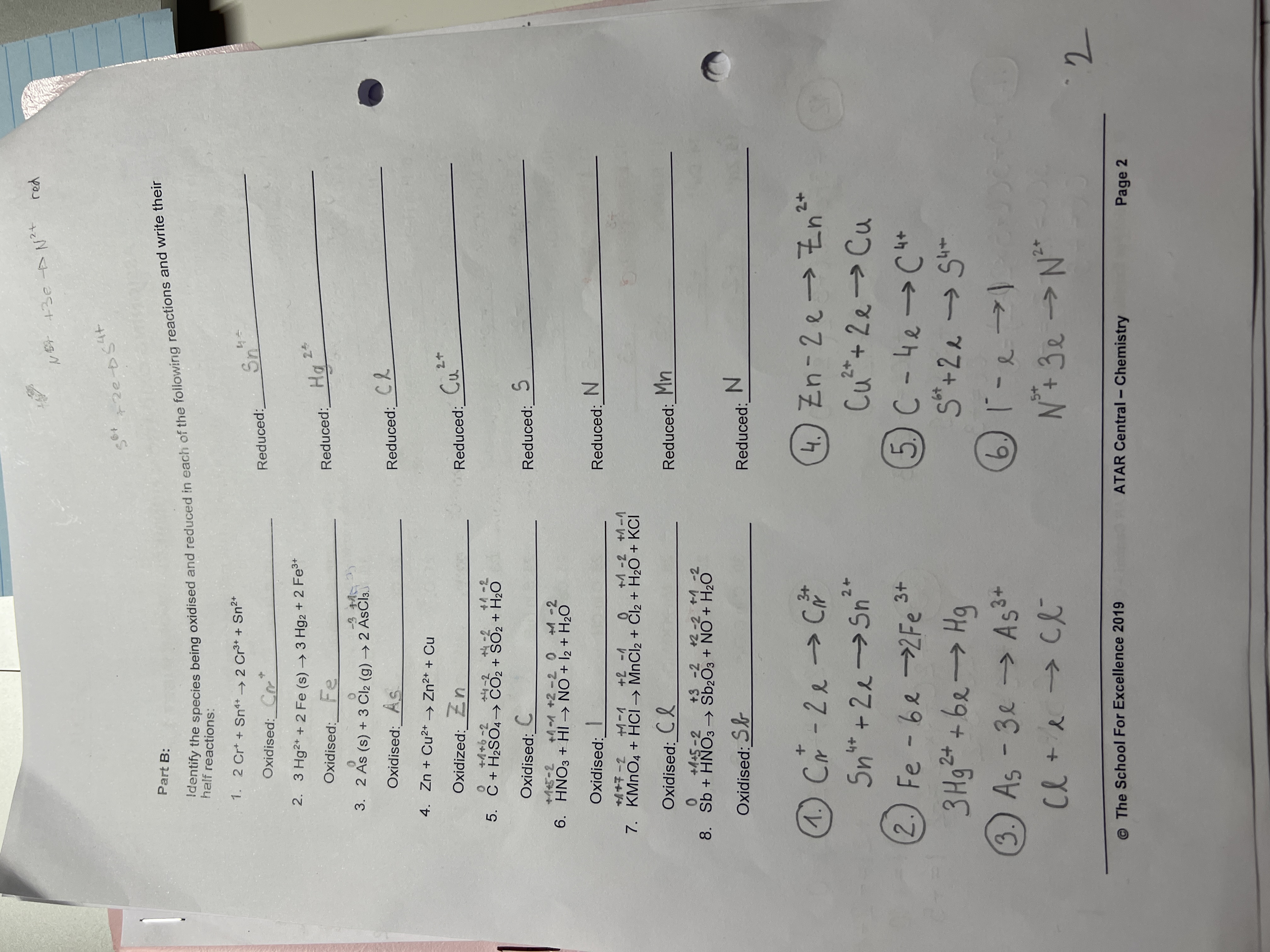
Identify the species being oxidised and reduced in each of the following reactions and write their half reactions.
Understand the Problem
The question is asking to identify the species being oxidized and reduced in various chemical reactions and to write their half reactions.
Answer
1. $Sn \rightarrow Sn^{2+} + 2e^-$; $Cu^{2+} + 2e^- \rightarrow Cu$ 2. $Fe \rightarrow Fe^{3+} + 3e^-$; $Hg^{2+} + 2e^- \rightarrow Hg$ 3. $As \rightarrow As^{3-} + 3e^-$; $Cl_2 + 2e^- \rightarrow 2Cl^-$ 4. $Zn \rightarrow Zn^{2+} + 2e^-$; $Cu^{2+} + 2e^- \rightarrow Cu$ 5. $C \rightarrow C^{4+} + 4e^-$; $S^{6+} + 2e^- \rightarrow S^{4+}$ 6. $I^- \rightarrow I + e^-$; $N^{3+} + 3e^- \rightarrow N^{2-}$
Answer for screen readers
-
Oxidized: $Sn \rightarrow Sn^{2+} + 2e^-$; Reduced: $Cu^{2+} + 2e^- \rightarrow Cu$
-
Oxidized: $Fe \rightarrow Fe^{3+} + 3e^-$; Reduced: $Hg^{2+} + 2e^- \rightarrow Hg$
-
Oxidized: $As \rightarrow As^{3-} + 3e^-$; Reduced: $Cl_2 + 2e^- \rightarrow 2Cl^-$
-
Oxidized: $Zn \rightarrow Zn^{2+} + 2e^-$; Reduced: $Cu^{2+} + 2e^- \rightarrow Cu$
-
Oxidized: $C \rightarrow C^{4+} + 4e^-$; Reduced: $S^{6+} + 2e^- \rightarrow S^{4+}$
-
Oxidized: $I^- \rightarrow I + e^-$; Reduced: $N^{3+} + 3e^- \rightarrow N^{2-}$
Steps to Solve
- Identify the Oxidation and Reduction Processes
In a redox reaction, oxidation is the loss of electrons while reduction is the gain of electrons. Identify which species are oxidized and which are reduced by analyzing changes in oxidation states.
- Write Half-Reactions for Oxidation
For each species identified as oxidized, write a half-reaction showing the loss of electrons. For example, Zn being oxidized to Zn²⁺ can be represented as:
$$ \text{Zn} \rightarrow \text{Zn}^{2+} + 2e^- $$
- Write Half-Reactions for Reduction
For each species identified as reduced, write a half-reaction showing the gain of electrons. For example, Cu²⁺ being reduced to Cu can be represented as:
$$ \text{Cu}^{2+} + 2e^- \rightarrow \text{Cu} $$
- Repeat for All Reactions
Continue to apply the above steps for each reaction provided, correctly identifying oxidized and reduced species, and writing their respective half-reactions.
- Check Consistency
Ensure that the number of electrons lost in oxidation is equal to the number of electrons gained in reduction for each reaction to maintain charge balance.
-
Oxidized: $Sn \rightarrow Sn^{2+} + 2e^-$; Reduced: $Cu^{2+} + 2e^- \rightarrow Cu$
-
Oxidized: $Fe \rightarrow Fe^{3+} + 3e^-$; Reduced: $Hg^{2+} + 2e^- \rightarrow Hg$
-
Oxidized: $As \rightarrow As^{3-} + 3e^-$; Reduced: $Cl_2 + 2e^- \rightarrow 2Cl^-$
-
Oxidized: $Zn \rightarrow Zn^{2+} + 2e^-$; Reduced: $Cu^{2+} + 2e^- \rightarrow Cu$
-
Oxidized: $C \rightarrow C^{4+} + 4e^-$; Reduced: $S^{6+} + 2e^- \rightarrow S^{4+}$
-
Oxidized: $I^- \rightarrow I + e^-$; Reduced: $N^{3+} + 3e^- \rightarrow N^{2-}$
More Information
Half-reactions are fundamental in balancing redox equations and understanding electron transfer processes. Each reaction reflects a change in oxidation states, enabling clear identification of oxidizing and reducing agents.
Tips
- Failing to properly balance charge and atoms in half-reactions.
- Misidentifying species as oxidized or reduced by not accurately tracking electron transfer.
- Overlooking that the loss of electrons in oxidation must equal the gain of electrons in reduction.
AI-generated content may contain errors. Please verify critical information