Understand the Problem
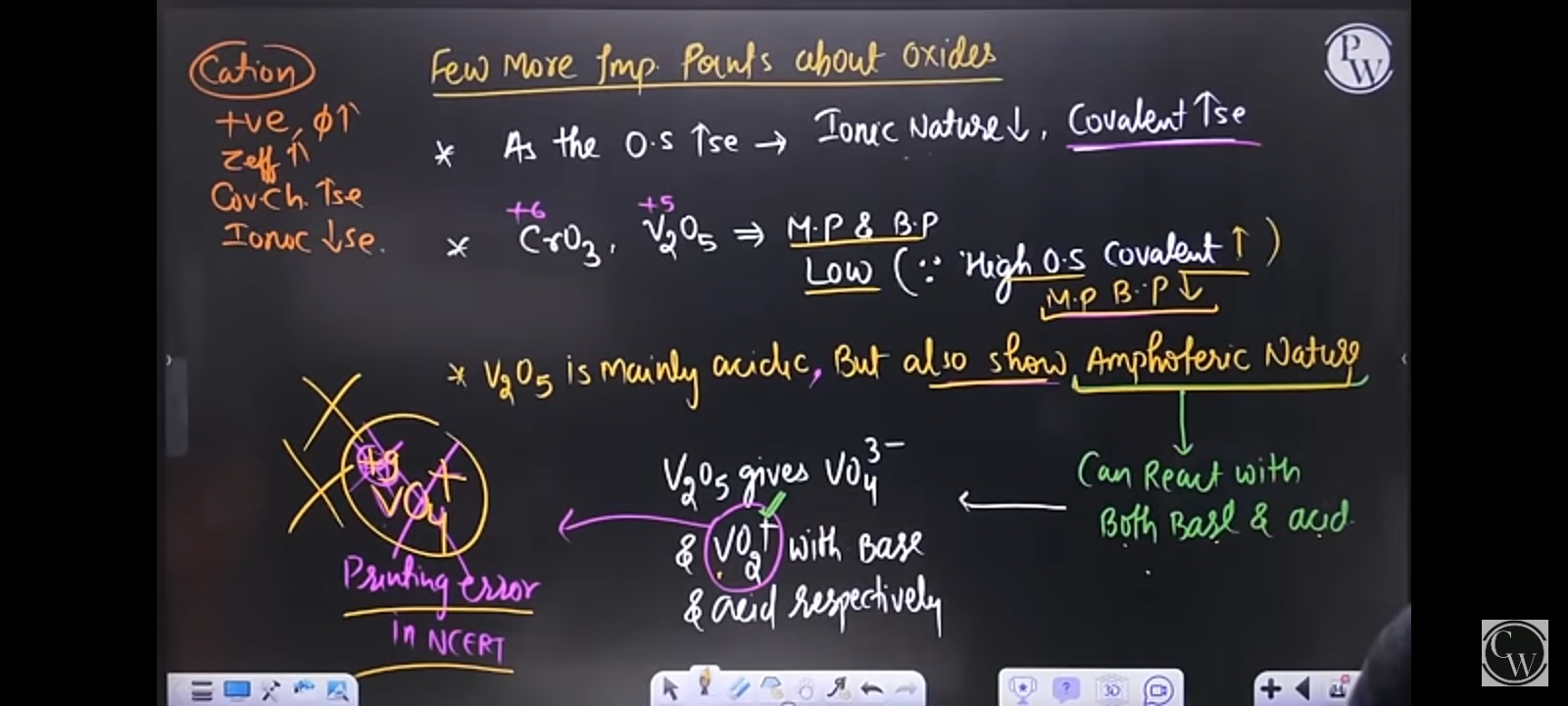
The image discusses properties of oxides, including their ionic and covalent nature, melting points, and acidic or amphoteric behavior. It explains how oxidation states influence these properties.
Answer
Higher oxidation states -> more covalent; V2O5 is both acidic and amphoteric.
The image explains the nature of oxides, indicating that as oxidation state increases, ionic character decreases and covalent character increases. V2O5 is acidic but also amphoteric, reacting with both acids and bases.
Answer for screen readers
The image explains the nature of oxides, indicating that as oxidation state increases, ionic character decreases and covalent character increases. V2O5 is acidic but also amphoteric, reacting with both acids and bases.
More Information
V2O5 is an interesting oxide because it can act as both an acid and a base, demonstrating its amphoteric properties. This characteristic is crucial for various chemical reactions and processes.
Tips
Ensure to identify whether an oxide is acidic, basic, or amphoteric based on its reaction behavior with acids and bases.
AI-generated content may contain errors. Please verify critical information