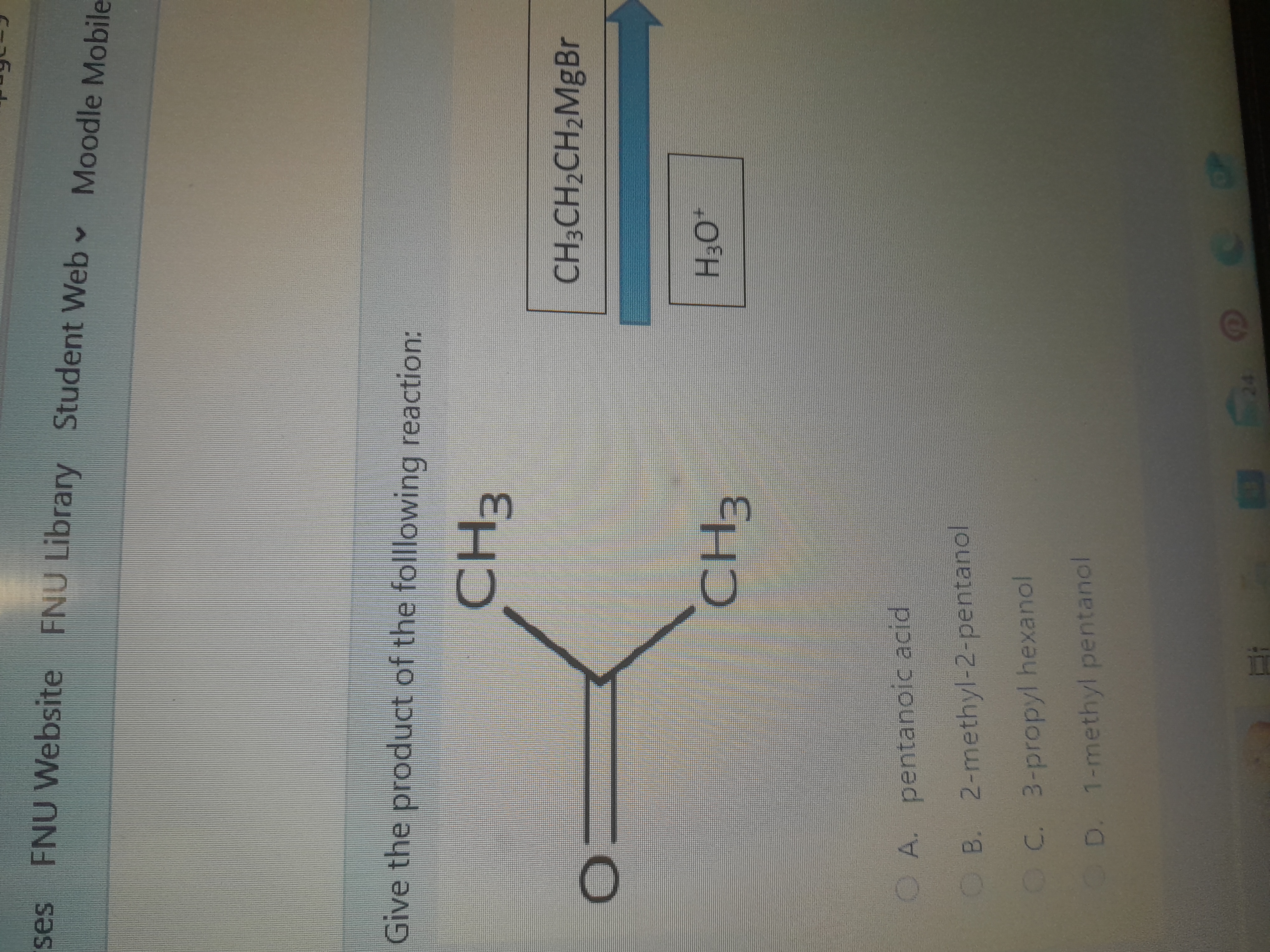
Give the product of the following reaction: CH3C(=O)CH3 + CH3CH2CH2MgBr → ? H3O+
Understand the Problem
The question is asking for the product of a chemical reaction involving a carbonyl compound and a Grignard reagent, followed by acid workup. This requires knowledge of organic chemistry to determine the expected product based on the given reactants.
Answer
The product is 3-propyl-2-pentanol.
Answer for screen readers
The product of the reaction is 3-propyl-2-pentanol.
Steps to Solve
- Identify the Reactants
The reactants are a carbonyl compound (specifically a ketone) represented as:
$$ \text{(CH}_3\text{)}_2\text{C=O} $$
and the Grignard reagent $ \text{CH}_3\text{CH}_2\text{CH}_2\text{MgBr} $.
- Understand Grignard Addition
Grignard reagents act as nucleophiles and react with carbonyl compounds. The carbonyl carbon is electrophilic, so the Grignard reagent will attack this carbon, resulting in an alkoxide intermediate.
- Formation of the Alkoxide
The reaction of the Grignard reagent with the ketone can be represented as:
$$ \text{(CH}_3\text{)}_2\text{C=O} + \text{CH}_3\text{CH}_2\text{CH}_2\text{MgBr} \rightarrow \text{(CH}_3\text{)}_2\text{C(OMgBr)(CH}_2\text{CH}_2\text{CH}_3)$$
- Acid Workup
After the alkoxide formation, the acid workup ($ \text{H}_3\text{O}^+ $) will protonate the alkoxide to give the final alcohol product:
$$ \text{(CH}_3\text{)}_2\text{C(OH)(CH}_2\text{CH}_2\text{CH}_3) $$
- Identify the Product Structure
The final product is a ternary alcohol with the structure of:
$$ \text{(CH}_3\text{)}_2\text{C}(\text{OH})\text{(CH}_2\text{CH}_2\text{CH}_3) $$
This compound can also be named as 3-propyl-2-pentanol (a six-carbon chain alcohol with a branching at the third carbon).
The product of the reaction is 3-propyl-2-pentanol.
More Information
When a Grignard reagent reacts with a carbonyl compound, it adds to the carbonyl carbon and forms an alcohol after acid workup. This mechanism is a fundamental reaction in organic chemistry for synthesizing alcohols.
Tips
- Confusing the order of the reactants and products in Grignard reactions.
- Forgetting that the product is formed after the acid workup, leading to incorrect structure identification.
- Not considering the branching structure that results from the addition of the Grignard reagent.
AI-generated content may contain errors. Please verify critical information