Find number of moles in 44 ml of water at STP.
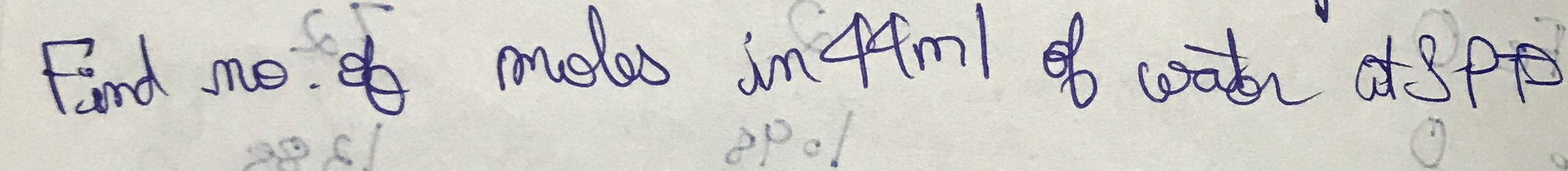
Understand the Problem
The question is asking to calculate the number of moles in 44 mL of water at standard pressure and temperature. This requires using the formula for moles, which is derived from the volume and the molar volume of water.
Answer
The number of moles in 44 mL of water at STP is approximately $2.44 \, \text{moles}$.
Answer for screen readers
The number of moles in 44 mL of water at STP is approximately $2.44 , \text{moles}$.
Steps to Solve
-
Identify the density of water At standard temperature and pressure (STP), the density of water is approximately $1 , \text{g/mL}$. This means that 1 mL of water weighs about 1 gram.
-
Calculate the mass of water To find the mass of 44 mL of water, use the formula: [ \text{Mass} = \text{Volume} \times \text{Density} ] Substituting the values, we get: [ \text{Mass} = 44 , \text{mL} \times 1 , \text{g/mL} = 44 , \text{grams} ]
-
Use the molar mass of water The molar mass of water ($\text{H}_2\text{O}$) is approximately $18 , \text{g/mol}$.
-
Calculate the number of moles To find the number of moles, use the formula: [ \text{Moles} = \frac{\text{Mass}}{\text{Molar Mass}} ] Substituting the mass of the water and its molar mass: [ \text{Moles} = \frac{44 , \text{grams}}{18 , \text{g/mol}} \approx 2.44 , \text{moles} ]
The number of moles in 44 mL of water at STP is approximately $2.44 , \text{moles}$.
More Information
The calculation demonstrates how to determine the number of moles from a given volume of water. The density and molar mass are critical in this process, emphasizing the relationship between volume, mass, and moles in chemistry.
Tips
- Confusing volume with mass: Always remember that volume should be converted to mass using density.
- Misidentifying the molar mass: It's essential to use the correct molar mass of the substance being evaluated. For water, the molar mass is about 18 g/mol, not 20 g/mol or others.
AI-generated content may contain errors. Please verify critical information