Explain osmosis and the difference between hypertonic, isotonic, and hypotonic solutions.
Understand the Problem
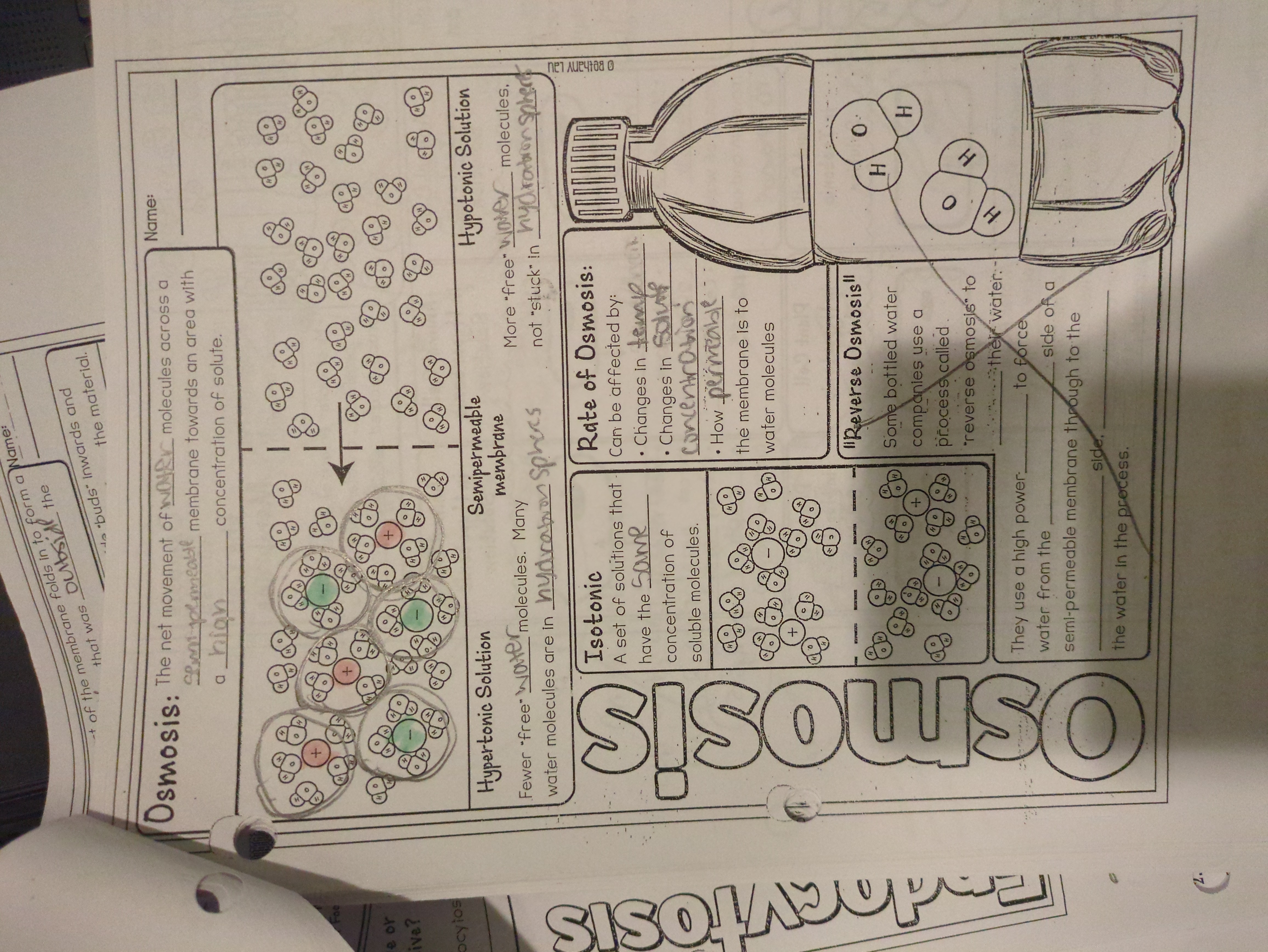
The question is focused on the concept of osmosis, specifically explaining how water molecules move through a semipermeable membrane and the differences between hypertonic, isotonic, and hypotonic solutions.
Answer
Osmosis: water moves across membranes. Hypertonic: cells shrink. Isotonic: cells stable. Hypotonic: cells swell.
Osmosis is the movement of water across a semipermeable membrane from low to high solute concentration. In hypertonic solutions, cells shrink as water exits. In isotonic solutions, cells remain unchanged as water flow is balanced. In hypotonic solutions, cells swell as water enters.
Answer for screen readers
Osmosis is the movement of water across a semipermeable membrane from low to high solute concentration. In hypertonic solutions, cells shrink as water exits. In isotonic solutions, cells remain unchanged as water flow is balanced. In hypotonic solutions, cells swell as water enters.
More Information
Osmosis is critical for maintaining cell homeostasis and is driven by differences in solute concentrations across the cell membrane.
Tips
Avoid confusing the direction of water movement: water always moves toward the higher solute concentration.
Sources
- Osmosis and Diffusion - khanacademy.org
- Hypotonic vs. Hypertonic vs. Isotonic: Learn The Difference - dictionary.com
AI-generated content may contain errors. Please verify critical information