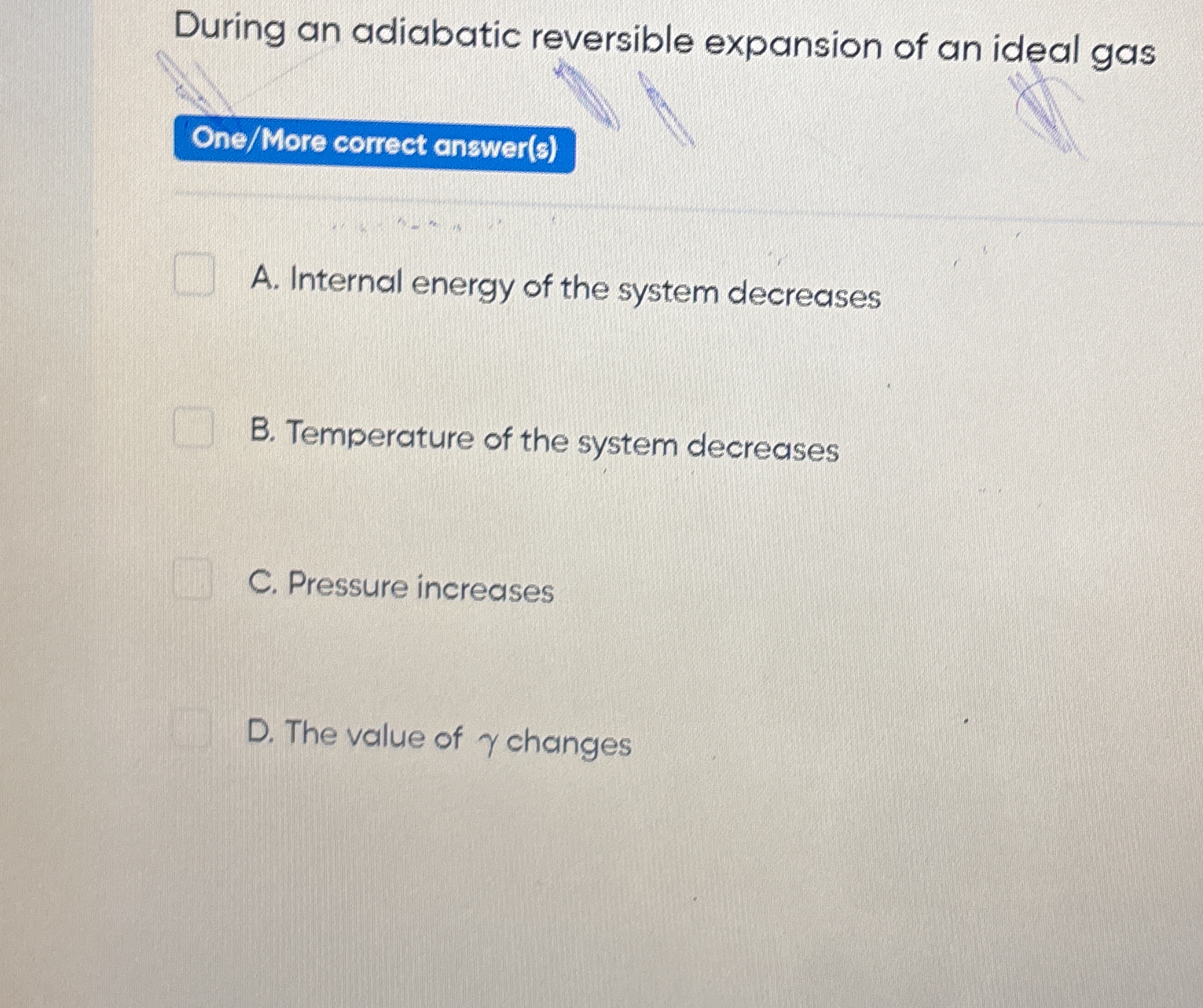
During an adiabatic reversible expansion of an ideal gas, what happens to the internal energy, temperature, pressure, and the value of γ?
Understand the Problem
The question is asking about the thermodynamic properties of an ideal gas during an adiabatic reversible expansion, specifically what happens to internal energy, temperature, pressure, and the value of gamma (γ).
Answer
Internal energy and temperature decrease; pressure decreases.
The internal energy and temperature of the system decrease, and pressure decreases. The value of γ does not change.
Answer for screen readers
The internal energy and temperature of the system decrease, and pressure decreases. The value of γ does not change.
More Information
During an adiabatic reversible expansion, work done by the gas decreases its internal energy and temperature. Pressure decreases due to increased volume and decreased temperature. γ remains constant.
Tips
A common mistake is thinking pressure increases; however, it decreases because the gas expands.
Sources
- Adiabatic Processes for an Ideal Gas - Physics LibreTexts - phys.libretexts.org
- Reversible Adiabatic Expansion of an Ideal Gas - phys.libretexts.org
AI-generated content may contain errors. Please verify critical information