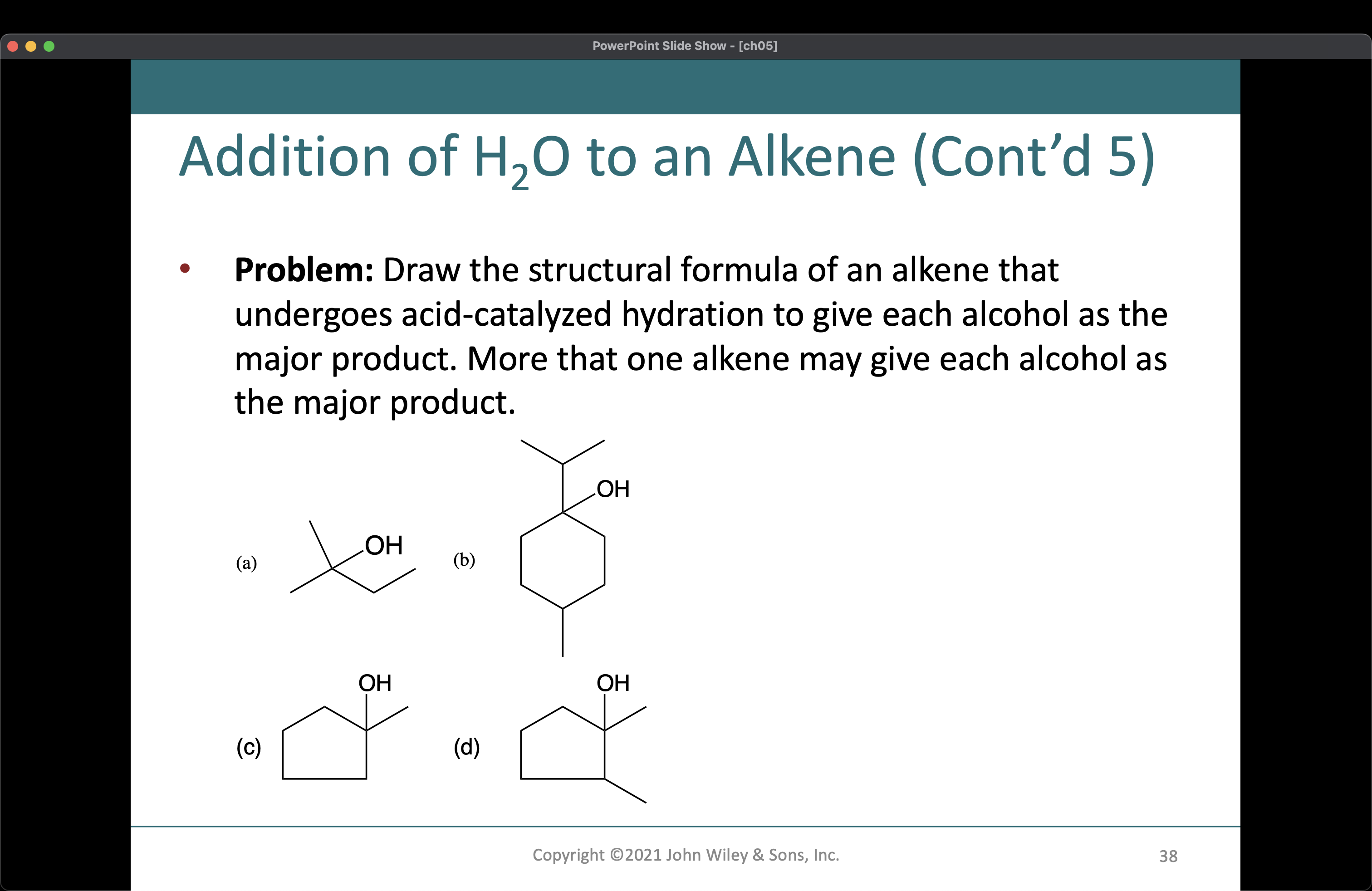
Draw the structural formula of an alkene that undergoes acid-catalyzed hydration to give each alcohol as the major product.
Understand the Problem
The question is asking for structural formulas of alkenes that undergo acid-catalyzed hydration to produce specified alcohols as major products. This involves drawing the chemical structure and understanding the reactions involved.
Answer
Alkenes: (a) 2-Butene, (b) 1-Methylcyclohexene, (c) Cyclopentene, (d) 2-Methyl-1-butene.
The alkenes for each alcohol are: (a) 2-Butene, (b) 1-Methylcyclohexene, (c) Cyclopentene, (d) 2-Methyl-1-butene.
Answer for screen readers
The alkenes for each alcohol are: (a) 2-Butene, (b) 1-Methylcyclohexene, (c) Cyclopentene, (d) 2-Methyl-1-butene.
More Information
Acid-catalyzed hydration often follows Markovnikov's rule, where the OH group attaches to the more substituted carbon.
Tips
A common mistake is neglecting possible rearrangements or unexpected shifts during the hydration process.
Sources
AI-generated content may contain errors. Please verify critical information