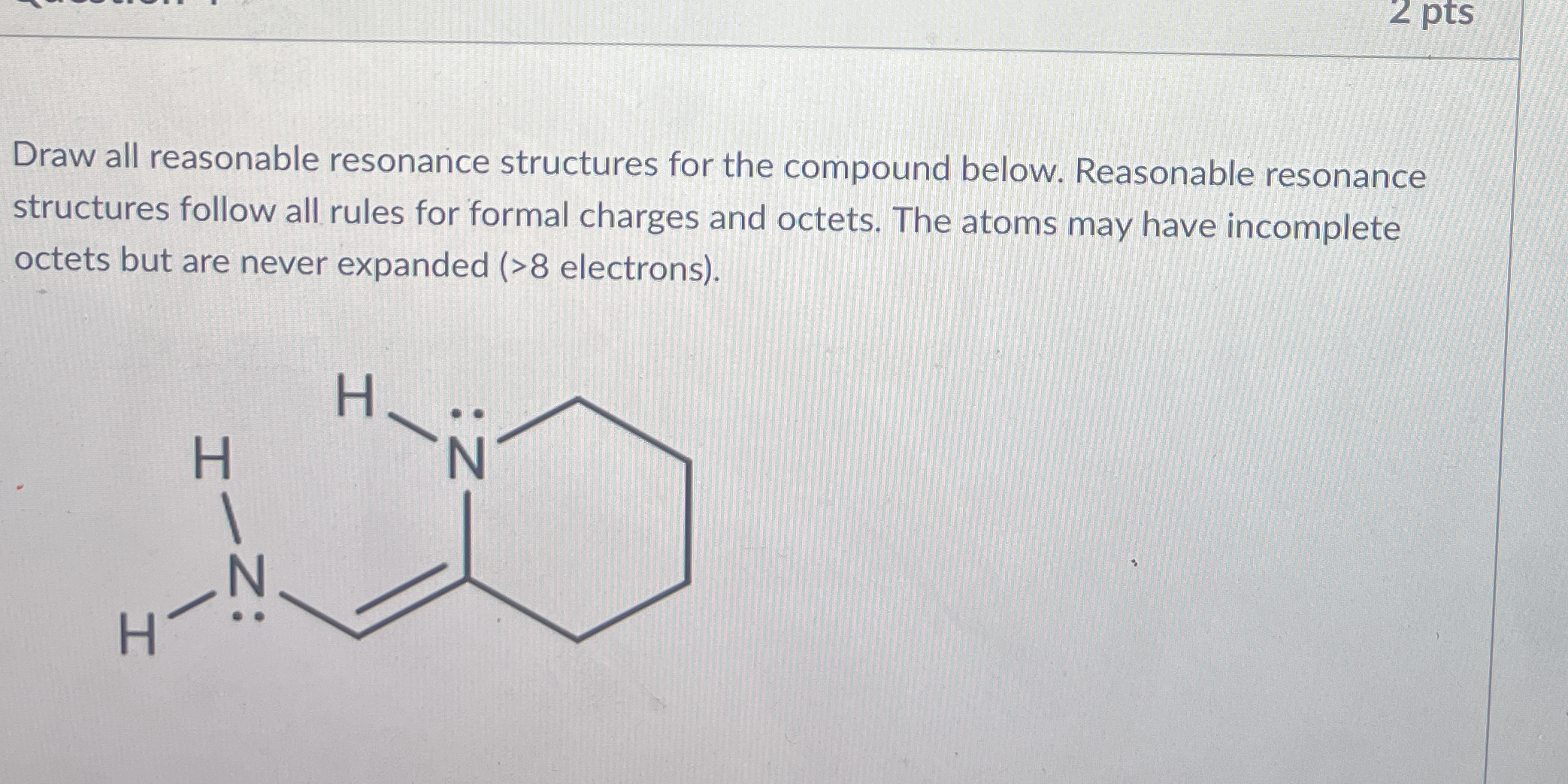
Draw all reasonable resonance structures for the compound below. Reasonable resonance structures follow all rules for formal charges and octets. The atoms may have incomplete octet... Draw all reasonable resonance structures for the compound below. Reasonable resonance structures follow all rules for formal charges and octets. The atoms may have incomplete octets but are never expanded (>8 electrons).
Understand the Problem
The question is asking for the identification and drawing of all reasonable resonance structures of a given chemical compound, ensuring that they adhere to formal charge and octet rules, while noting that atoms can have incomplete octets but cannot have expanded octets.
Answer
Shift electrons to form resonance structures involving N atoms and double bonds.
The compound's resonance structures involve the shifting of electrons between the nitrogen atoms and the adjacent double-bonded carbon, forming structures like aza-pyridazine, where differing nitrogen atoms carry the positive charge. Ensure all structures are valid with respect to formal charges and the octet rule.
Answer for screen readers
The compound's resonance structures involve the shifting of electrons between the nitrogen atoms and the adjacent double-bonded carbon, forming structures like aza-pyridazine, where differing nitrogen atoms carry the positive charge. Ensure all structures are valid with respect to formal charges and the octet rule.
More Information
Resonance structures help represent the delocalization of electrons in compounds, contributing to stability.
Tips
Avoid exceeding the octet on nitrogen and verify formal charges.
Sources
- Rules for Resonance Forms - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information