Distinguish the difference between the different orders of reaction and apply accordingly.
Understand the Problem
The question is asking to differentiate between different orders of reactions in applied chemistry, specifically focusing on pseudo-order reactions and the rate of reactions. It also includes various problems related to first and second order reactions.
Answer
Reaction order describes how reactant concentration influences reaction rate.
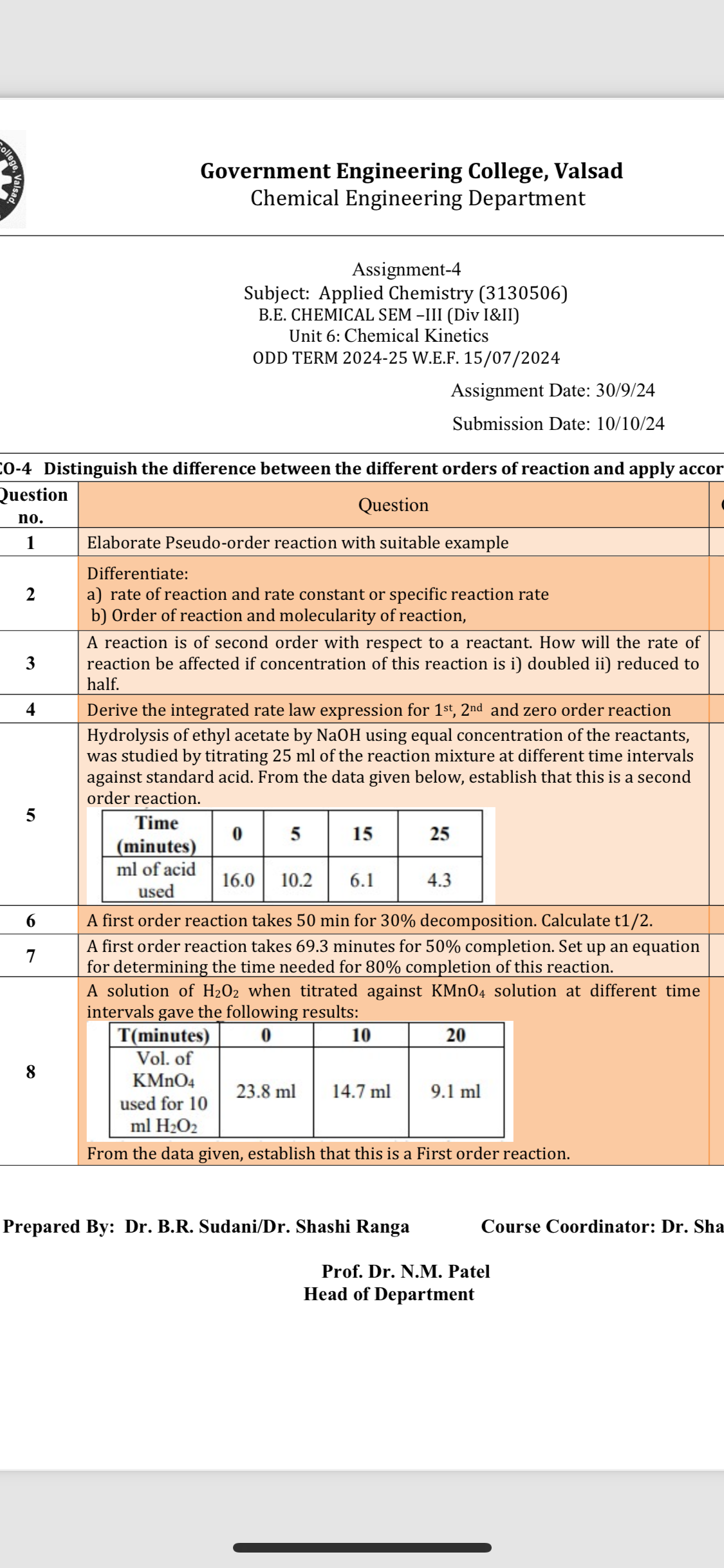
The final answer is that reaction orders determine how reactant concentrations affect reaction rates. Zero-order: rate is constant. First-order: rate is proportional to concentration of one reactant. Second-order: rate depends on concentration squared or two reactants linearly.
Answer for screen readers
The final answer is that reaction orders determine how reactant concentrations affect reaction rates. Zero-order: rate is constant. First-order: rate is proportional to concentration of one reactant. Second-order: rate depends on concentration squared or two reactants linearly.
More Information
Reaction order is crucial for predicting how changes in concentrations affect reaction speeds and for determining reaction mechanisms.
Tips
A common mistake is confusing rate with rate constant. Rate depends on concentration, while rate constant is fixed at a given temperature.
Sources
- Reaction Order - First & Second Order - ChemTalk - chemistrytalk.org
- 3.3.3: Reaction Order - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information