Distinguish between carbocation and carbanion. Write IUPAC names of the following compounds: i) nitrobenzene ii) aniline. Define Redox reaction and explain with a suitable example.... Distinguish between carbocation and carbanion. Write IUPAC names of the following compounds: i) nitrobenzene ii) aniline. Define Redox reaction and explain with a suitable example. Draw resonating structures of aniline. Write Stock notation of the following compounds: i) CrO3 ii) SnCl4.
Understand the Problem
The question asks for several chemistry-related concepts to be distinguished or explained. It specifically looks for definitions, structures, and chemical names related to organic chemistry and redox reactions.
Answer
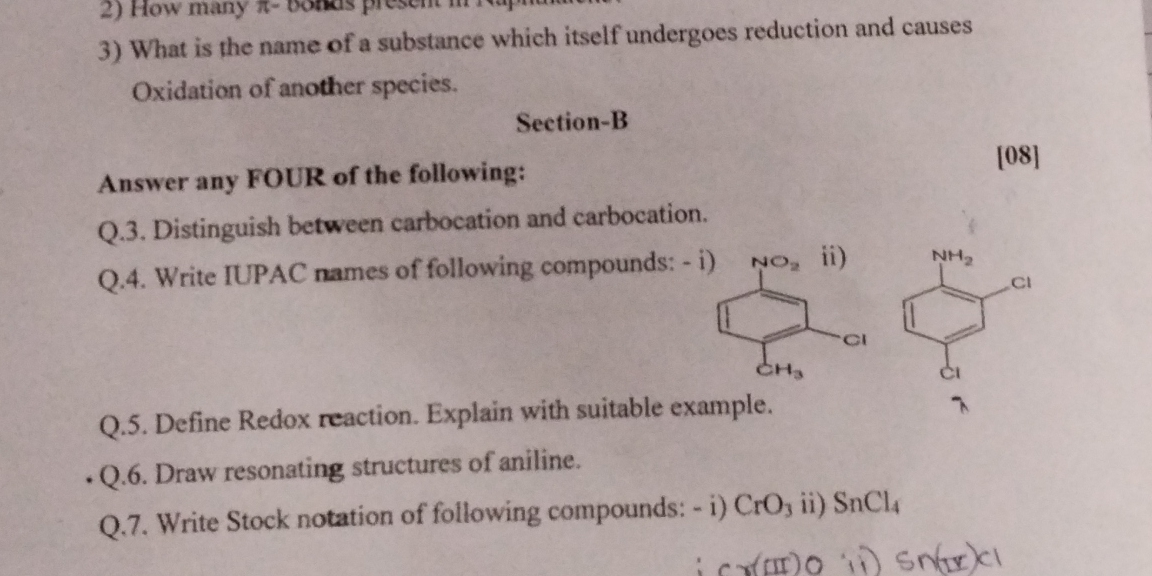
Carbocations: +ve, Carbanions: -ve; IUPAC: 1-Chloro-4-methyl-3-nitrobenzene, 2,4-Dichloroaniline; Redox: Electron transfer; Cr(VI)O3, Sn(IV)Cl4.
Distinguish: Carbocations are positive, reactive, and unstable; carbanions are negative and nucleophilic. IUPAC Names: i) 1-Chloro-4-methyl-3-nitrobenzene ii) 2,4-Dichloroaniline. Redox: Electron transfer reactions, e.g., rusting of iron. Resonating Structures: Various canonical forms by electron pair shifting. Stock Notation: Cr(VI)O3, Sn(IV)Cl4.
Answer for screen readers
Distinguish: Carbocations are positive, reactive, and unstable; carbanions are negative and nucleophilic. IUPAC Names: i) 1-Chloro-4-methyl-3-nitrobenzene ii) 2,4-Dichloroaniline. Redox: Electron transfer reactions, e.g., rusting of iron. Resonating Structures: Various canonical forms by electron pair shifting. Stock Notation: Cr(VI)O3, Sn(IV)Cl4.
More Information
Carbocations and carbanions play important roles in organic reactions, such as electrophilic and nucleophilic reactions, respectively. Redox reactions are essential in energy transformations, such as those in batteries and biological systems.
Tips
Don't confuse carbocation (positive) with carbanion (negative). Make sure resonance structures accurately reflect electron movement.
AI-generated content may contain errors. Please verify critical information