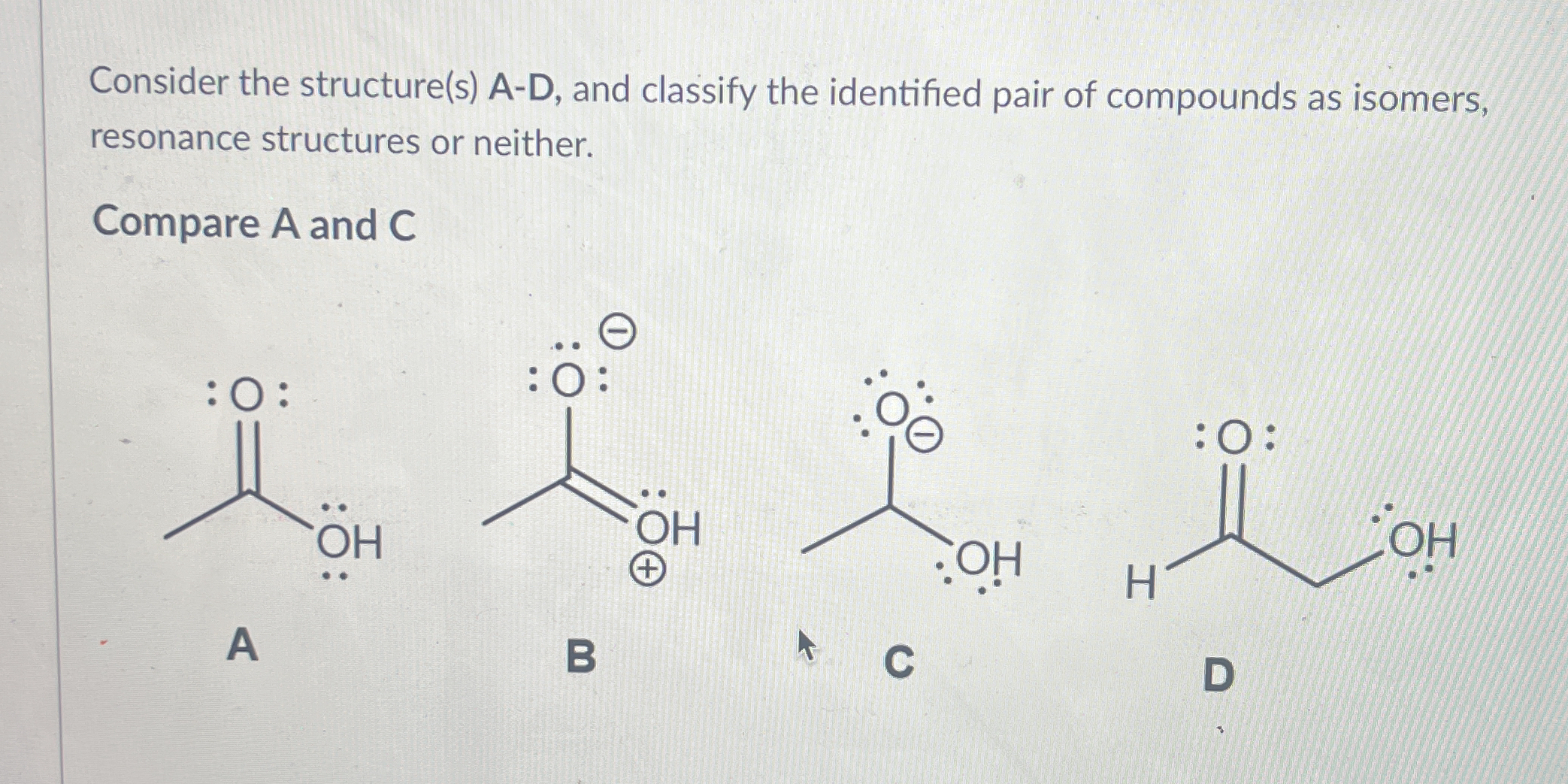
Consider the structure(s) A-D, and classify the identified pair of compounds as isomers, resonance structures or neither. Compare A and C.
Understand the Problem
The question is asking to analyze the chemical structures labeled A and C, and classify them either as isomers, resonance structures, or neither based on their configurations.
Answer
Isomers
The final answer is that structures A and C are isomers.
Answer for screen readers
The final answer is that structures A and C are isomers.
More Information
Isomers are compounds with the same molecular formula but different connectivity of atoms. Structures A and C have the same molecular formula but different atomic arrangements.
Tips
Ensure to differentiate between the connectivity of atoms (isomers) and mere electron shifting (resonance).
AI-generated content may contain errors. Please verify critical information