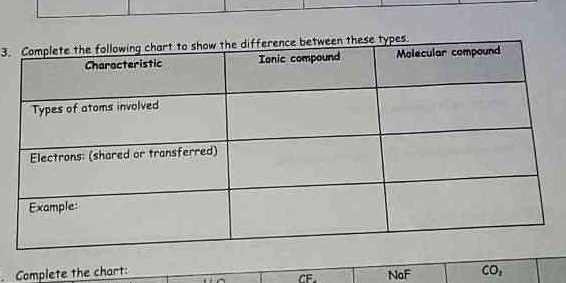
Complete the following chart to show the difference between ionic compound and molecular compound based on characteristics such as types of atoms involved, electrons shared or tran... Complete the following chart to show the difference between ionic compound and molecular compound based on characteristics such as types of atoms involved, electrons shared or transferred, and examples.
Understand the Problem
The question is asking to complete a chart that compares ionic and molecular compounds based on certain characteristics, such as types of atoms, electron interaction, and examples.
Answer
Ionic: metals & nonmetals, electrons transferred, e.g., NaCl. Molecular: nonmetals, electrons shared, e.g., CO2.
For ionic compounds, metals and nonmetals are involved, electrons are transferred, and examples include NaCl and MgO. For molecular compounds, nonmetals are involved, electrons are shared, with examples like CO2 and H2O.
Answer for screen readers
For ionic compounds, metals and nonmetals are involved, electrons are transferred, and examples include NaCl and MgO. For molecular compounds, nonmetals are involved, electrons are shared, with examples like CO2 and H2O.
More Information
Ionic compounds are typically solid and have high melting points. Molecular compounds can be gases, liquids, or solids with lower melting points.
Tips
Ensure proper distinction between types of atoms (metals vs. nonmetals) and behaviors of electrons (transferred vs. shared).
Sources
- 3.1: Ionic and Molecular Compounds - Chemistry LibreTexts - chem.libretexts.org
- Molecular and Ionic Compounds | Chemistry for Majors - courses.lumenlearning.com
AI-generated content may contain errors. Please verify critical information