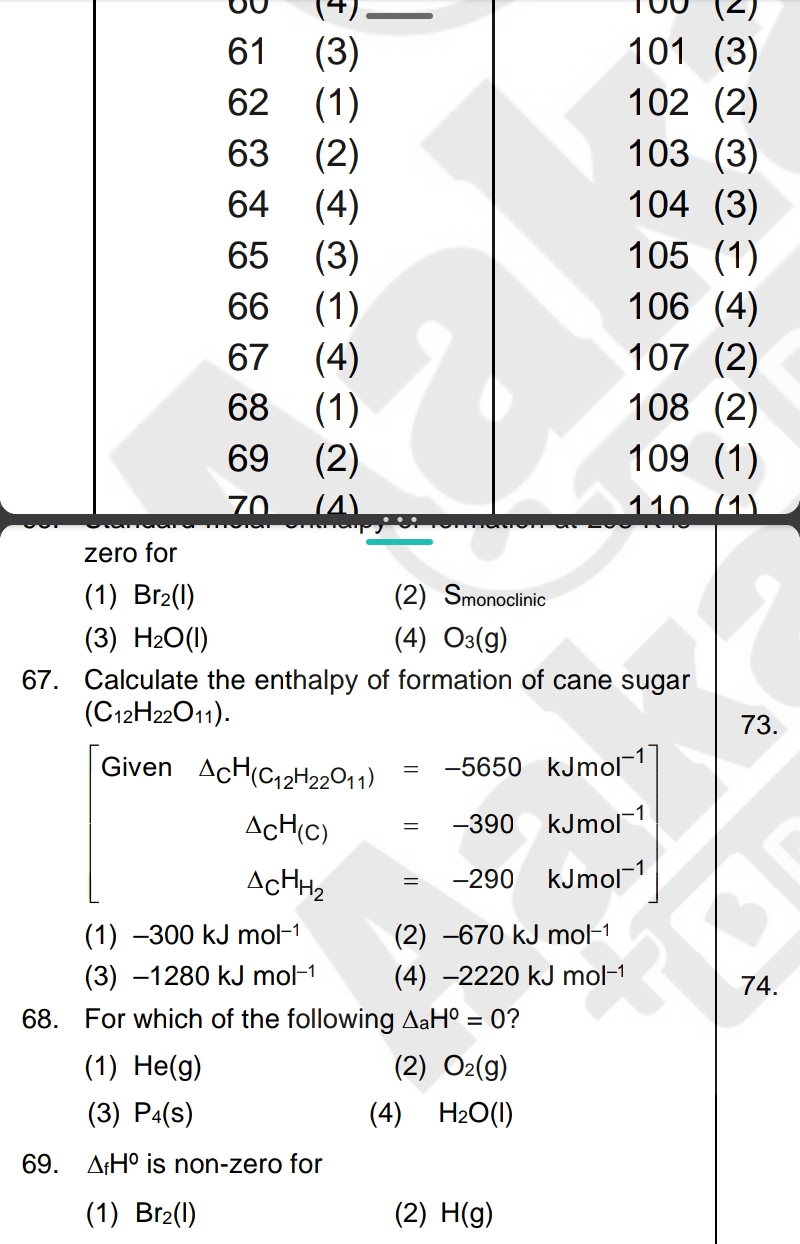
Calculate the enthalpy of formation of cane sugar (C12H22O11). Given: ΔH(C12H22O11) = -5650 kJ mol^-1, ΔH(C) = -390 kJ mol^-1, ΔH(H2) = -290 kJ mol^-1.
Understand the Problem
The question involves calculating the enthalpy of formation of cane sugar (C12H22O11) using provided data. It also queries regarding standard enthalpy values and conditions for different substances.
Answer
-2220 kJ/mol
The enthalpy of formation of cane sugar is -2220 kJ/mol.
Answer for screen readers
The enthalpy of formation of cane sugar is -2220 kJ/mol.
More Information
The enthalpy of formation provides information about the energy change when one mole of a compound forms from its elements in their standard states.
Tips
Ensure to correctly convert units when necessary and use the proper stoichiometric coefficients.
Sources
AI-generated content may contain errors. Please verify critical information