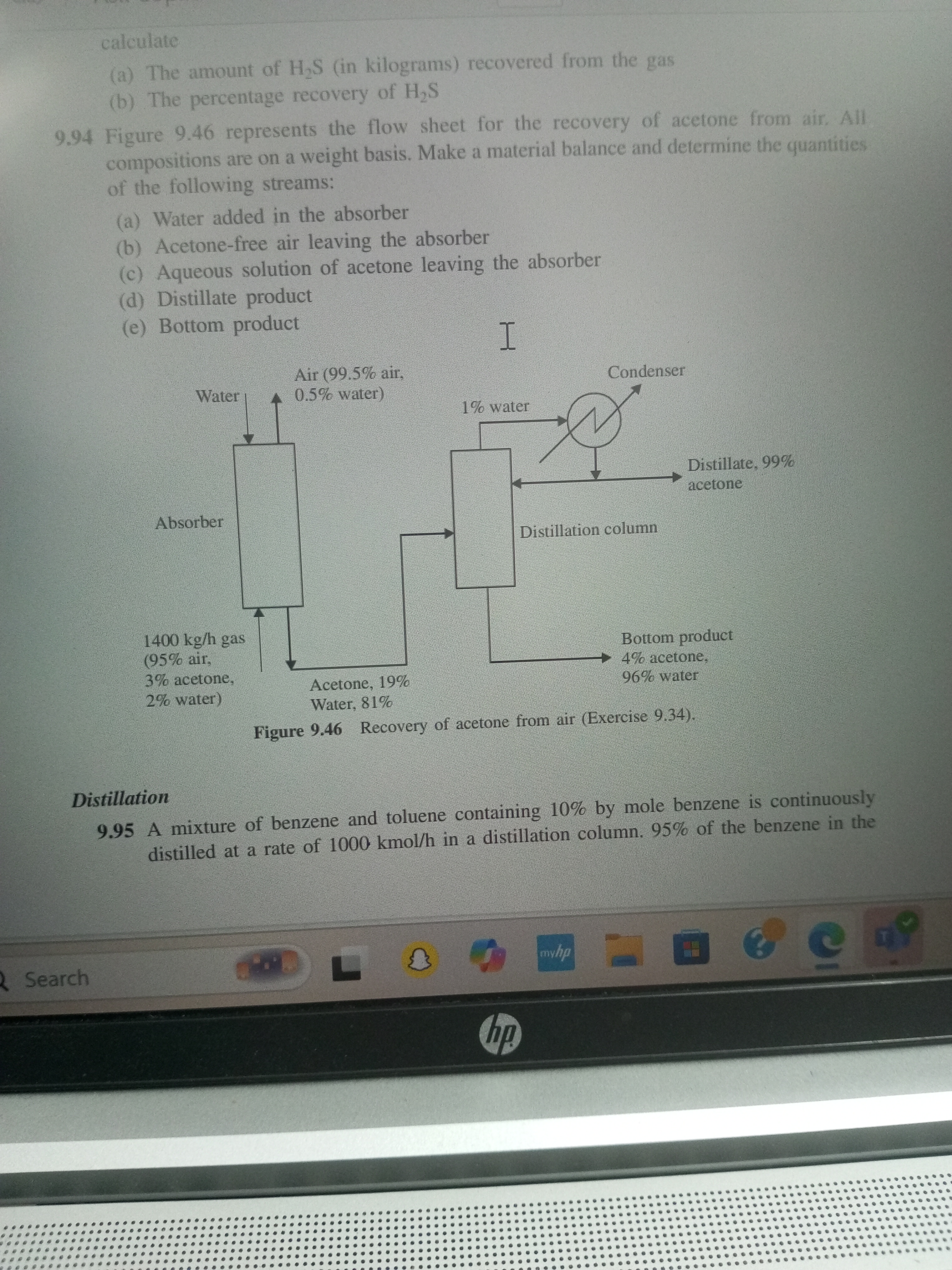
Calculate (a) the amount of H₂S (in kilograms) recovered from the gas, (b) the percentage recovery of H₂S. Make a material balance and determine the quantities of the following str... Calculate (a) the amount of H₂S (in kilograms) recovered from the gas, (b) the percentage recovery of H₂S. Make a material balance and determine the quantities of the following streams: (a) Water added in the absorber, (b) Acetone-free air leaving the absorber, (c) Aqueous solution of acetone leaving the absorber, (d) Distillate product, (e) Bottom product.
Understand the Problem
The question is asking for calculations related to the recovery of H₂S and the flow sheet representing the recovery of acetone from air. Specifically, it requests the amount of H₂S recovered and the percentage recovery, as well as various quantities in the flow sheet related to water, air, and acetone solutions.
Answer
The amount of H₂S recovered is based on the calculations derived from acetone recovery, and the percentage recovery can be computed with the total input of H₂S.
Answer for screen readers
(a) The amount of H₂S recovered (in kg) is calculated based on the acetone recovery.
(b) The percentage recovery of H₂S is determined using the formula provided.
Steps to Solve
- Define the Input and Output Streams
Identify the input and output streams mentioned in the problem.
- Input: 1400 kg/h gas containing 3% acetone and 2% water.
- Output: Streams with specified compositions (distillate and bottom products).
- Calculate the Input Composition
Calculate the total amount of each component in the input gas stream.
For acetone in the input: $$ \text{Acetone} = 0.03 \times 1400 , \text{kg/h} = 42 , \text{kg/h} $$
For water in the input: $$ \text{Water} = 0.02 \times 1400 , \text{kg/h} = 28 , \text{kg/h} $$
- Identify the Components in Output Streams
Define the output streams from the flow sheet:
- Distillate product (99% acetone): Let the amount be $D$ kg/h.
- Bottom product (4% acetone): Let the amount be $B$ kg/h.
- Material Balance for Acetone
Set up the material balance for acetone:
$$ 42 = 0.99D + 0.04B $$
- Material Balance for Water
Set up the material balance for water:
$$ 28 = 0.01D + 0.96B $$
- Solving the Equations
Now, solve the system of equations for $D$ and $B$.
From the first equation: $$ B = \frac{42 - 0.99D}{0.04} $$
Substitute this $B$ into the water balance equation.
- Calculate the Amount of H₂S Recovered
If the H₂S recovery pertains to the flow of acetone, use the relationship between acetone and H₂S to find the recovery in kg.
- Calculate the Percentage Recovery of H₂S
The percentage recovery can be calculated by: $$ \text{Percentage Recovery} = \left( \frac{\text{Recovered H₂S}}{\text{Input H₂S}} \right) \times 100 $$
(a) The amount of H₂S recovered (in kg) is calculated based on the acetone recovery.
(b) The percentage recovery of H₂S is determined using the formula provided.
More Information
The calculations involve balancing the input and output streams to determine various quantities and can help in the design of separation processes.
Tips
- Failing to properly account for all components in the material balance.
- Misinterpreting the flow rates or percentages indicated in the diagram.
- Not substituting correctly when solving simultaneous equations.
AI-generated content may contain errors. Please verify critical information