Based on Figure 1, which of the following best describes how the properties of water at an air-water interface enable an insect to walk on the water's surface?
Understand the Problem
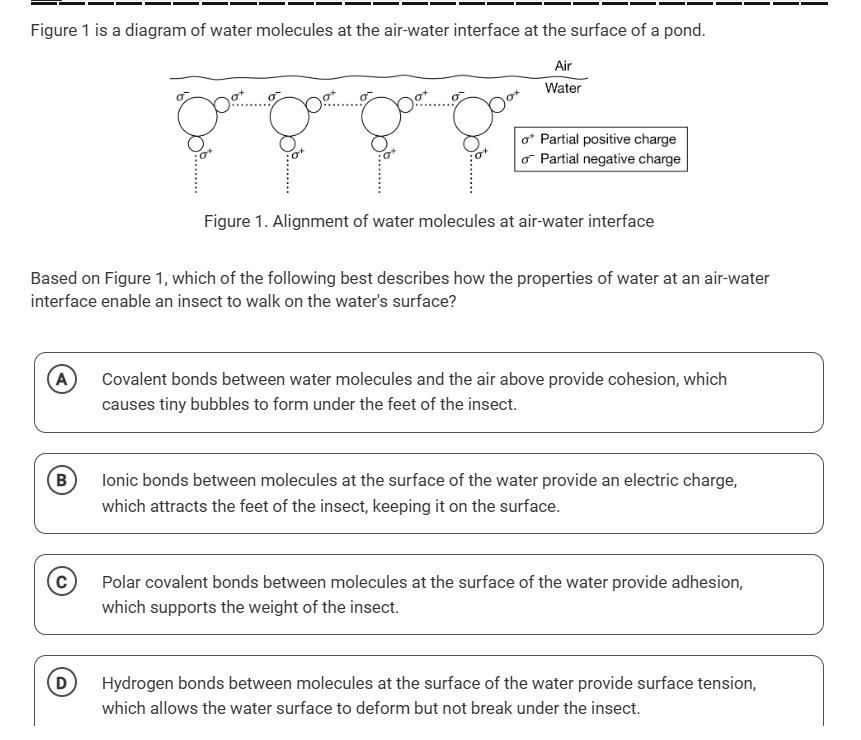
The question is asking how the properties of water at the air-water interface allow an insect to walk on the water's surface, based on the provided diagram of water molecules. It presents multiple-choice answers that describe different types of molecular interactions.
Answer
Hydrogen bonds between molecules at the surface of the water provide surface tension, which allows the water surface to deform but not break under the insect.
The final answer is D) Hydrogen bonds between molecules at the surface of the water provide surface tension, which allows the water surface to deform but not break under the insect.
Answer for screen readers
The final answer is D) Hydrogen bonds between molecules at the surface of the water provide surface tension, which allows the water surface to deform but not break under the insect.
More Information
Insects are able to walk on the surface of water due to the surface tension created by hydrogen bonds between water molecules. This surface tension acts like a 'skin' on the water's surface, allowing the insect to stay on top without breaking through.
Tips
A common mistake is to confuse hydrogen bonds with covalent or ionic bonds when explaining the properties of water surfaces. Remember that surface tension is primarily due to hydrogen bonds.
Sources
AI-generated content may contain errors. Please verify critical information