Balance the following redox reaction under acidic aqueous conditions using the smallest whole-number coefficients possible. What is the coefficient of HSO3-? MnO4-(aq) + HSO3-(aq)... Balance the following redox reaction under acidic aqueous conditions using the smallest whole-number coefficients possible. What is the coefficient of HSO3-? MnO4-(aq) + HSO3-(aq) → Mn2+(aq) + SO3^(2-)(aq)
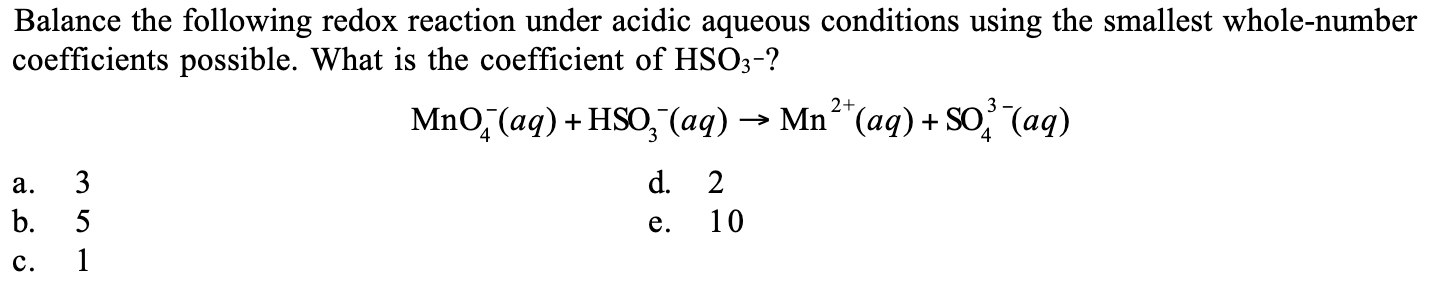
Understand the Problem
The question is asking to balance a redox reaction and determine the coefficient of HSO3- in the balanced equation. This involves identifying the oxidation and reduction states, and applying stoichiometric methods to find the correct coefficients.
Answer
5
Answer for screen readers
The coefficient of $HSO_3^{-}$ in the balanced equation is 5.
Steps to Solve
- Identify Oxidation States Determine the oxidation states of each element in the reactants and products:
- In $MnO_4^-$, Mn is in the +7 oxidation state.
- In $HSO_3^-$, S is in the +4 oxidation state.
- In $Mn^{2+}$, Mn is in the +2 oxidation state.
- In $SO_3^{2-}$, S is still in the +4 oxidation state.
- Determine Oxidation and Reduction Identify what is being oxidized and what is being reduced:
- Mn is reduced from +7 to +2 (gaining electrons).
- S is not oxidized or reduced—it changes oxidation state but remains the same in terms of total electrons.
- Write Half-Reactions Write the half-reactions for oxidation and reduction:
- Reduction half-reaction for Mn: $$ MnO_4^{-} + 8H^+ + 5e^- \rightarrow Mn^{2+} + 4H_2O $$
- Oxidation half-reaction for HSO3- (acting as a reducing agent): $$ 2HSO_3^{-} \rightarrow 2SO_3^{2-} + 2H^+ + 2e^- $$
- Balance Electrons Make sure the number of electrons lost equals the number of electrons gained:
- Multiply the oxidation half-reaction by 5 to balance electrons: $$ 5(2HSO_3^{-} \rightarrow 2SO_3^{2-} + 2H^+ + 2e^-) $$ This leads to: $$ 10HSO_3^{-} \rightarrow 10SO_3^{2-} + 10H^+ + 10e^- $$
-
Combine Half-Reactions Add the balanced half-reactions together: $$ MnO_4^{-} + 10HSO_3^{-} + 8H^+ + 5e^- \rightarrow Mn^{2+} + 4H_2O + 10SO_3^{2-} + 10H^+ + 10e^- $$
-
Simplify the Reaction Combine and simplify to get the final balanced reaction: $$ MnO_4^{-} + 5HSO_3^{-} + 4H^+ \rightarrow Mn^{2+} + 5SO_3^{2-} + 2H_2O $$
The coefficient of $HSO_3^{-}$ in the balanced equation is 5.
More Information
This reaction demonstrates the principles of redox reactions where one species is reduced while the other is oxidized, emphasizing the conservation of mass and charge.
Tips
- Forgetting to balance both mass and charge in each half-reaction.
- Overlooking the need to adjust coefficients in either half-reaction to equalize the electrons gained and lost.
AI-generated content may contain errors. Please verify critical information