Balance the following equations and identify the type of reaction: NaNO3 + PbO → Pb(NO3)2 + Na2O.
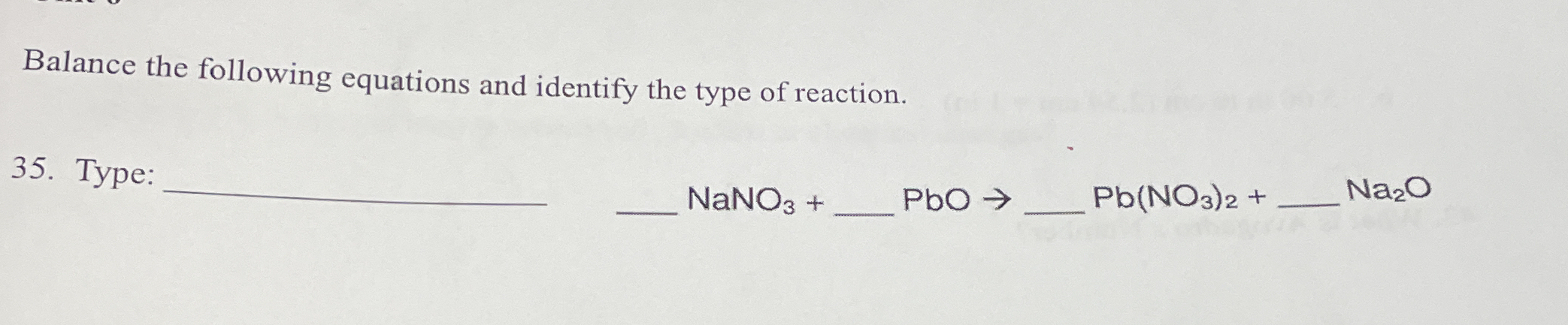
Understand the Problem
The question is asking to balance a chemical equation involving sodium nitrate (NaNO3) and lead(II) oxide (PbO), and to identify the type of chemical reaction represented by this equation.
Answer
The balanced equation is $2 \text{NaNO}_3 + \text{PbO} \rightarrow \text{Pb(NO}_3\text{)}_2 + \text{Na}_2\text{O}$. This is a double displacement reaction.
Answer for screen readers
The balanced equation is: $$ 2 \text{NaNO}_3 + \text{PbO} \rightarrow \text{Pb(NO}_3\text{)}_2 + \text{Na}_2\text{O} $$
Steps to Solve
-
Identify Reactants and Products The reactants are sodium nitrate ($\text{NaNO}_3$) and lead(II) oxide ($\text{PbO}$). The products are lead(II) nitrate ($\text{Pb(NO}_3\text{)}_2$) and sodium oxide ($\text{Na}_2\text{O}$).
-
Count Atoms on Both Sides Count the number of each type of atom in the unbalanced equation:
-
Reactants:
- Na: 1
- N: 1
- O: 3 (from NaNO3) + 1 (from PbO) = 4
- Pb: 1
-
Products:
- Pb: 1
- N: 2 (from Pb(NO3)2)
- O: 6 (from Pb(NO3)2) + 1 (from Na2O) = 7
- Na: 2
-
Reactants:
-
Balance Sodium (Na) To balance sodium, place a coefficient of 2 before $\text{NaNO}_3$: $$ 2 \text{NaNO}_3 + \text{PbO} \rightarrow \text{Pb(NO}_3\text{)}_2 + \text{Na}_2\text{O} $$
-
Recount Atoms After Balancing Sodium Now recount the atoms:
-
Reactants:
- Na: 2
- N: 2
- O: 6 (from 2NaNO3) + 1 (from PbO) = 7
- Pb: 1
-
Products:
- Pb: 1
- N: 2
- O: 6 (from Pb(NO3)2) + 1 (from Na2O) = 7
- Na: 2
-
Reactants:
-
Confirm Balance Now both sides have:
- Na: 2
- N: 2
- O: 7
- Pb: 1
The equation is now balanced.
- Identify the Type of Reaction This reaction is a double displacement reaction because it involves the exchange of ions between two compounds.
The balanced equation is: $$ 2 \text{NaNO}_3 + \text{PbO} \rightarrow \text{Pb(NO}_3\text{)}_2 + \text{Na}_2\text{O} $$
More Information
In double displacement reactions, compounds exchange ions to form new products. This type of reaction often occurs in aqueous solutions. The balanced equation indicates that two moles of sodium nitrate react with one mole of lead(II) oxide to produce one mole of lead(II) nitrate and one mole of sodium oxide.
Tips
- Not balancing ion charges: Ensure that ionic species have the same charge before balancing.
- Overlooking coefficients: Double-check that coefficients are correctly applied to all species involved.
AI-generated content may contain errors. Please verify critical information