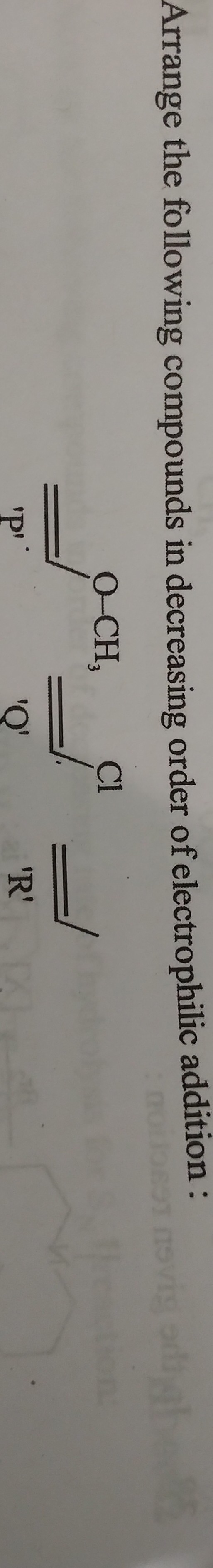
Arrange the following compounds in decreasing order of electrophilic addition.
Understand the Problem
The question is asking to arrange three chemical compounds labeled 'P', 'Q', and 'R' in order of their reactivity towards electrophilic addition. This involves analyzing the structures of these compounds to determine their relative reactivity based on factors like electron density and stability.
Answer
The order of reactivity is $R > P > Q$.
Answer for screen readers
The compounds in decreasing order of reactivity towards electrophilic addition are:
$$ R > P > Q $$
Steps to Solve
-
Identify the Compounds Identify the structures of compounds P, Q, and R based on the image provided. Compound P has an alkene containing a methoxy group, compound Q is an alkene, and compound R is an alkyne.
-
Assess Reactivity Based on Structure Analyze the electron density in the double and triple bonds:
- Compounds with double bonds (alkenes) will generally react via electrophilic addition.
- Compounds with triple bonds (alkynes) have higher electron density and generally react more readily than alkenes.
-
Consider Substituent Effects Consider the influence of any substituents:
- In compound P, the methoxy group ($-OCH_3$) is electron-donating through resonance, increasing the electron density and reactivity towards electrophiles.
- Compound Q does not have electron-donating or withdrawing groups affecting the double bond.
- Compound R, being an alkyne, is more reactive than alkenes.
-
Rank the Compounds by Reactivity Based on the analysis:
- Alkyne (compound R) > Alkene with strong electron-donating group (compound P) > Alkene without strong substituents (compound Q).
-
Final Order of Reactivity Arrange the compounds in decreasing order of their reactivity towards electrophilic addition.
The compounds in decreasing order of reactivity towards electrophilic addition are:
$$ R > P > Q $$
More Information
This arrangement is based on the electron-donating effects of substituents and the inherent reactivity of alkenes versus alkynes. Alkynes are typically more reactive due to their higher electron density, coupled with the presence of electron-donating groups that can further enhance reactivity.
Tips
- Misjudging the effect of substituents: It's important to consider how electron-donating or withdrawing groups affect reactivity.
- Confusing alkene and alkyne reactivity; remember that alkynes are generally more reactive than alkenes in electrophilic addition reactions.
AI-generated content may contain errors. Please verify critical information