Answer the paper.
Understand the Problem
The question is about answering a chemistry exam paper for a class, which includes various multiple-choice questions on topics such as valency, chemical reactions, and formulas.
Answer
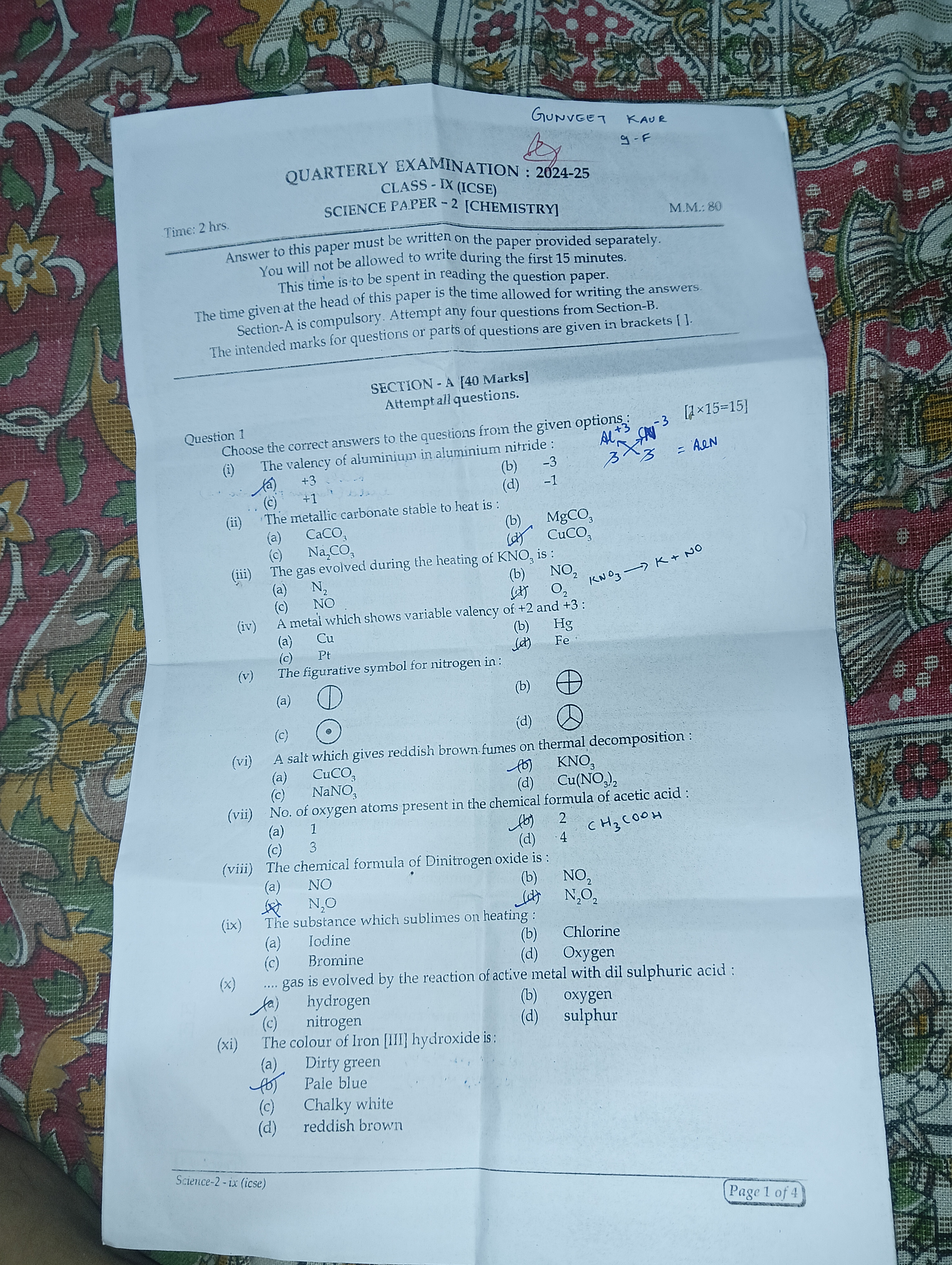
["(i) +3","(ii) CaCO\u2083","(iii) NO\u2082","(iv) Fe","(v) c","(vi) KNO\u2083","(vii) 4","(viii) N\u2082O","(ix) Iodine","(x) Nitrogen","(xi) Dirty green"]
["1. (i) Answer: +3 (Aluminium in aluminium nitride, AlN)","1. (ii) Answer: CaCO\u2083 (The metallic carbonate stable to heat)","1. (iii) Answer: NO\u2082 (Gas evolved during heating of KNO\u2083)","1. (iv) Answer: Fe (Metal with variable valency)","1. (v) Answer: c (Circled dot represents Nitrogen)","1. (vi) Answer: KNO\u2083 (Salt giving reddish brown fumes on thermal decomposition)","1. (vii) Answer: 4 (Number of oxygen atoms in acetic acid, C\u2082H\u2084O\u2082)","1. (viii) Answer: N\u2082O (Chemical formula of Dinitrogen oxide)","1. (ix) Answer: Iodine (Substance subliming on heating)","1. (x) Answer: Nitrogen (Gas evolved by active metals with dilute sulphuric acid)","1. (xi) Answer: Dirty green (Colour of Iron [II] Hydroxide)"]
Answer for screen readers
["1. (i) Answer: +3 (Aluminium in aluminium nitride, AlN)","1. (ii) Answer: CaCO\u2083 (The metallic carbonate stable to heat)","1. (iii) Answer: NO\u2082 (Gas evolved during heating of KNO\u2083)","1. (iv) Answer: Fe (Metal with variable valency)","1. (v) Answer: c (Circled dot represents Nitrogen)","1. (vi) Answer: KNO\u2083 (Salt giving reddish brown fumes on thermal decomposition)","1. (vii) Answer: 4 (Number of oxygen atoms in acetic acid, C\u2082H\u2084O\u2082)","1. (viii) Answer: N\u2082O (Chemical formula of Dinitrogen oxide)","1. (ix) Answer: Iodine (Substance subliming on heating)","1. (x) Answer: Nitrogen (Gas evolved by active metals with dilute sulphuric acid)","1. (xi) Answer: Dirty green (Colour of Iron [II] Hydroxide)"]
More Information
This sheet covers a range of chemistry topics including valency, reaction gases, and properties of metals. Avoiding mistakes often requires revisiting textbook sections corresponding to each question.
Tips
Common mistakes include misunderstanding the chemical formulae or valencies of elements. Review formulae and valencies carefully to avoid simple errors.
Sources
- Paper Homework Help & Answers - Studypool - studypool.com
AI-generated content may contain errors. Please verify critical information