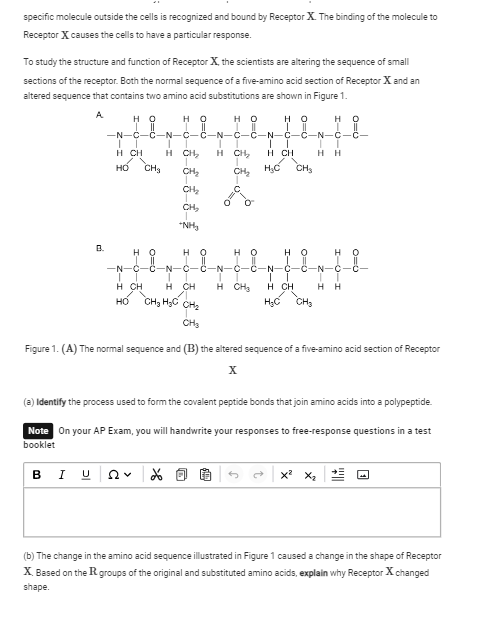
(a) Identify the process used to form the covalent peptide bonds that join amino acids into a polypeptide. (b) The change in the amino acid sequence illustrated in Figure 1 caused... (a) Identify the process used to form the covalent peptide bonds that join amino acids into a polypeptide. (b) The change in the amino acid sequence illustrated in Figure 1 caused a change in the shape of Receptor X. Based on the R groups of the original and substituted amino acids, explain why Receptor X changed shape.
Understand the Problem
The question is asking to identify the process of peptide bond formation in proteins and to explain how changes in amino acid sequences can affect protein shape and function, focusing on Receptor X.
Answer
(a) Dehydration synthesis. (b) Altered R groups affected folding and interactions in Receptor X.
(a) The process used is dehydration synthesis (or condensation reaction). (b) Receptor X changed shape because the different R groups in the substituted amino acids altered the way the polypeptide folded, affecting interactions like hydrogen bonds and hydrophobic/hydrophilic interactions.
Answer for screen readers
(a) The process used is dehydration synthesis (or condensation reaction). (b) Receptor X changed shape because the different R groups in the substituted amino acids altered the way the polypeptide folded, affecting interactions like hydrogen bonds and hydrophobic/hydrophilic interactions.
More Information
Dehydration synthesis involves the removal of a water molecule to form a peptide bond between amino acids. The shape of proteins depends heavily on the interactions and properties of the R groups, such as charge, polarity, and size, which affect folding.
Tips
One common mistake is to ignore the role of R group properties in protein folding. Always consider how changes in charge or size can impact protein structure.
AI-generated content may contain errors. Please verify critical information