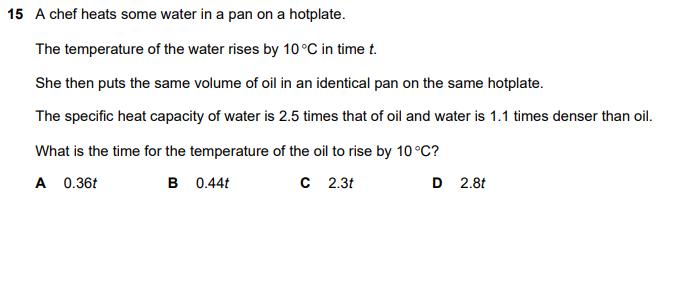
A chef heats some water in a pan on a hotplate. The temperature of the water rises by 10°C in time t. She then puts the same volume of oil in an identical pan on the same hotplate.... A chef heats some water in a pan on a hotplate. The temperature of the water rises by 10°C in time t. She then puts the same volume of oil in an identical pan on the same hotplate. The specific heat capacity of water is 2.5 times that of oil and water is 1.1 times denser than oil. What is the time for the temperature of the oil to rise by 10°C?
Understand the Problem
The problem describes a scenario where water and oil are heated on a hotplate. Given the time it takes for water to increase by 10°C, and the relationships concerning specific heat capacity and density between water and oil, the objective is to compute the duration for an equivalent volume of oil to rise by 10°C on the same hotplate. This will require using the heat equation Q=mcΔT and the information about density and specific heat to find the relationship bewteen the time it takes to heat oil compared to water.
Answer
A. $0.36t$
Answer for screen readers
A. $0.36t$
Steps to Solve
- Define the heat equation
The heat $Q$ required to change the temperature of a substance is given by: $Q = mc\Delta T$ where $m$ is the mass, $c$ is the specific heat capacity, and $\Delta T$ is the change in temperature.
- Relate heat to time
Since the hotplate is the same, the rate of heat transfer is constant. Therefore, the heat supplied is proportional to time: $Q \propto t$ $Q = kt$, where $k$ is a constant.
- Express mass in terms of density and volume
Density $\rho$ is mass per unit volume: $\rho = \frac{m}{V}$ So, $m = \rho V$ We can substitute this into the heat equation: $Q = \rho V c \Delta T$
- Apply the equation to water and oil
For water: $Q_w = \rho_w V c_w \Delta T_w$ For oil: $Q_o = \rho_o V c_o \Delta T_o$
- Express the time in terms of density, specific heat, and temperature change
Since $Q = kt$: For water: $kt_w = \rho_w V c_w \Delta T_w$ For oil: $kt_o = \rho_o V c_o \Delta T_o$
- Find the ratio of times
Divide the equation for oil by the equation for water: $\frac{t_o}{t_w} = \frac{\rho_o V c_o \Delta T_o}{\rho_w V c_w \Delta T_w}$ Since the volume $V$ and temperature change $\Delta T$ are the same for both water and oil, they cancel out: $\frac{t_o}{t_w} = \frac{\rho_o c_o}{\rho_w c_w}$
- Use the given relationships
We are given that $c_w = 2.5c_o$ and $\rho_w = 1.1\rho_o$. Substitute these into the equation above: $\frac{t_o}{t_w} = \frac{\rho_o c_o}{1.1\rho_o \cdot 2.5c_o} = \frac{1}{1.1 \cdot 2.5} = \frac{1}{2.75}$
- Solve for $t_o$
$t_o = \frac{t_w}{2.75} = \frac{1}{2.75}t_w \approx 0.36t_w$ Since $t_w = t$, then $t_o \approx 0.36t$
A. $0.36t$
More Information
The problem combines concepts from thermodynamics and heat transfer, specifically dealing with specific heat capacity, density, and the relationship between heat, mass, and temperature change.
Tips
A common mistake would be to not correctly use the ratios for the specific heat capacity and density. For example, using $c_o = 2.5 c_w$ instead of $c_w = 2.5 c_o$. Another common mistake is not using the density when calculating heat which leads to an incorrect answer.
AI-generated content may contain errors. Please verify critical information