Understand the Problem
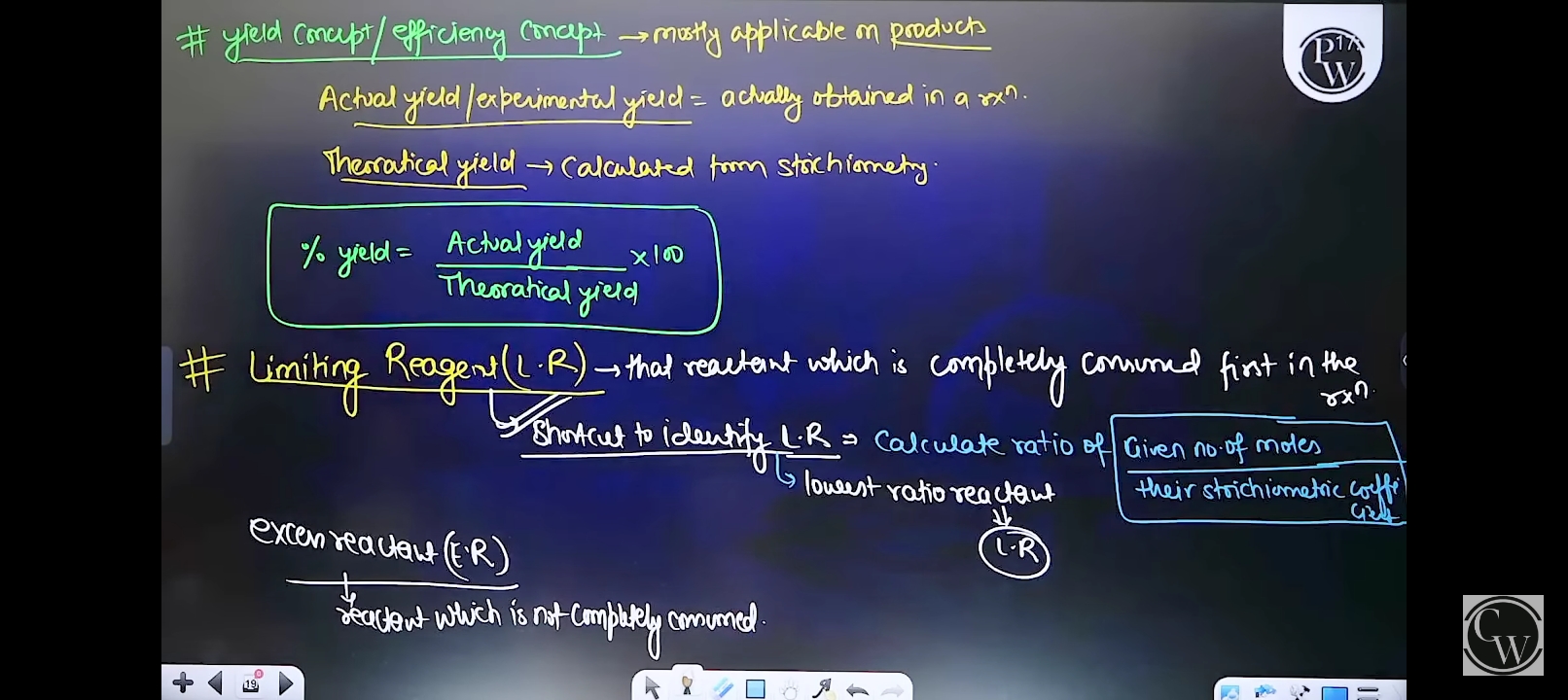
The image discusses concepts related to yield in chemistry, including actual yield, theoretical yield, percent yield, and limiting reagents. It explains how to calculate yield and identify limiting reagents, which are essential concepts in stoichiometry.
Answer
Explains percent yield, limiting reagent, excess reagent concepts.
The final answer explains the concepts of percent yield, limiting reagent, and excess reagent.
Answer for screen readers
The final answer explains the concepts of percent yield, limiting reagent, and excess reagent.
More Information
Percent yield indicates efficiency in a chemical reaction. Limiting reagent is the reactant that determines the maximum product formed.
Tips
A common mistake is confusing the limiting reagent with the reactant in excess. Ensure correct stoichiometric ratios are used.
AI-generated content may contain errors. Please verify critical information