Understand the Problem
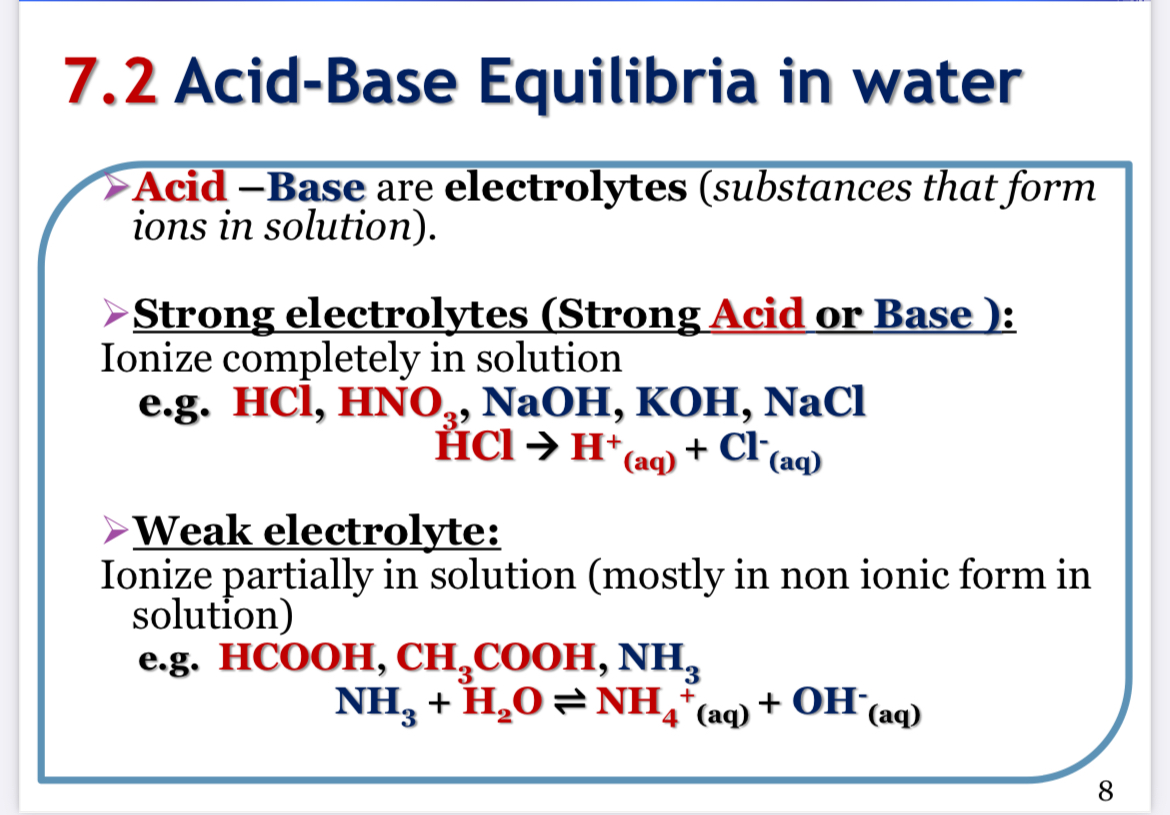
The question appears to be presenting information on the concepts of acid-base equilibria, specifically differentiating between strong and weak electrolytes and their behavior in solution. It covers definitions, examples, and ionization processes.
Answer
Strong electrolytes ionize completely; weak electrolytes partially ionize.
The final answer is about distinguishing strong and weak electrolytes.
Answer for screen readers
The final answer is about distinguishing strong and weak electrolytes.
More Information
Strong electrolytes, such as HCl and NaOH, dissociate fully in solution, producing ions. Weak electrolytes, like acetic acid (CH3COOH), only partially dissociate.
Tips
A common mistake is confusing complete ionization with partial ionization. Always check if the electrolyte is strong or weak.
AI-generated content may contain errors. Please verify critical information