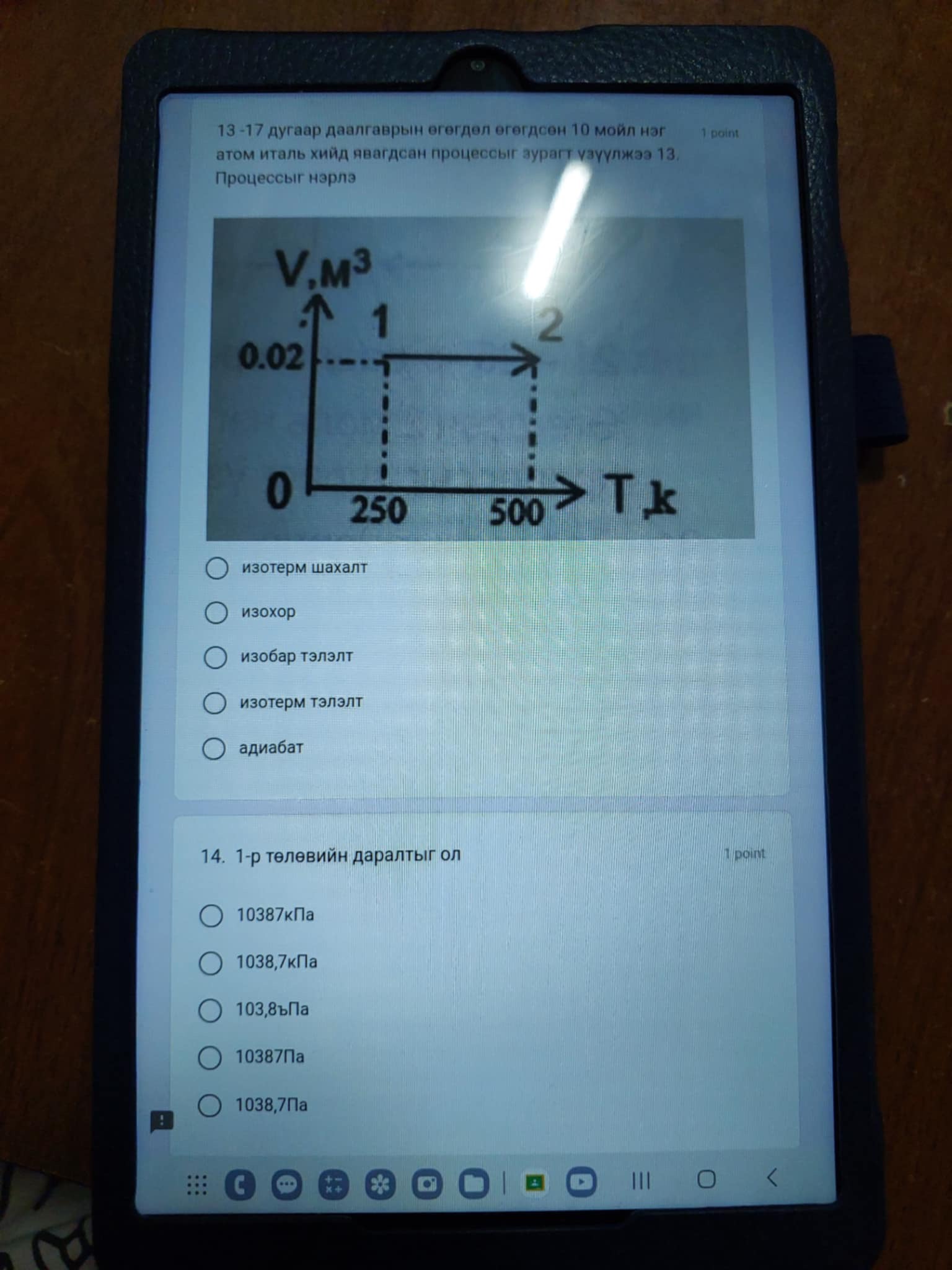
10 моль нэг атомт мөсөн усны процессын график өгөгдлөө. Таны харж байгаа графикаар аль процесс явагдаж байгааг тодорхойл. 10 моль нэг атомт мөсөн усны процессын график өгөгдлөө. Таны харж байгаа графикаар аль процесс явагдаж байгааг тодорхойл.
Understand the Problem
Сурагчдаас эсрэг процессийг зураг дээр тайлбарлахыг асууж байна. Зураг дээрх графикаар процессыг тодорхойлж, зөв хариуг олж гаргах шаардлагатай.
Answer
Isothermal process.
Answer for screen readers
The process represented is an isothermal process.
Steps to Solve
-
Process Type Identification
The graph depicts a process involving gas or fluid. Since the volume is changing while the temperature remains constant from points 1 to 2, this indicates that the process is isothermal. -
Understanding Isothermal Process
An isothermal process occurs at a constant temperature, implying that any internal energy changes are offset by heat exchanges with the surroundings. In the graph, the horizontal line shows that volume is increasing while temperature does not change. -
Equation for Isothermal Process
For an ideal gas under isothermal conditions, the relationship can be expressed by the equation:
$$ P_1 V_1 = P_2 V_2 $$
Where (P) is pressure, and (V) is volume. -
Calculating Pressure
Given that the process is isothermal, we can apply the ideal gas law and observe that we can calculate pressure at state 1 or state 2 using the pressures and volumes at these states if provided. -
Final Pressure Determination
Select the correct pressure value from the options provided after making calculations. The required pressure value will depend on the specific volume and temperature values according to the ideal gas law.
The process represented is an isothermal process.
More Information
An isothermal process is crucial in thermodynamics as it helps understand energy exchanges during gas behavior at constant temperatures. It also plays a fundamental role in heat engines and refrigeration cycles.
Tips
- Confusing isothermal with other processes such as isobaric (constant pressure) or adiabatic (no heat exchange).
- Misinterpreting the graph scale or values leading to incorrect calculations.
AI-generated content may contain errors. Please verify critical information