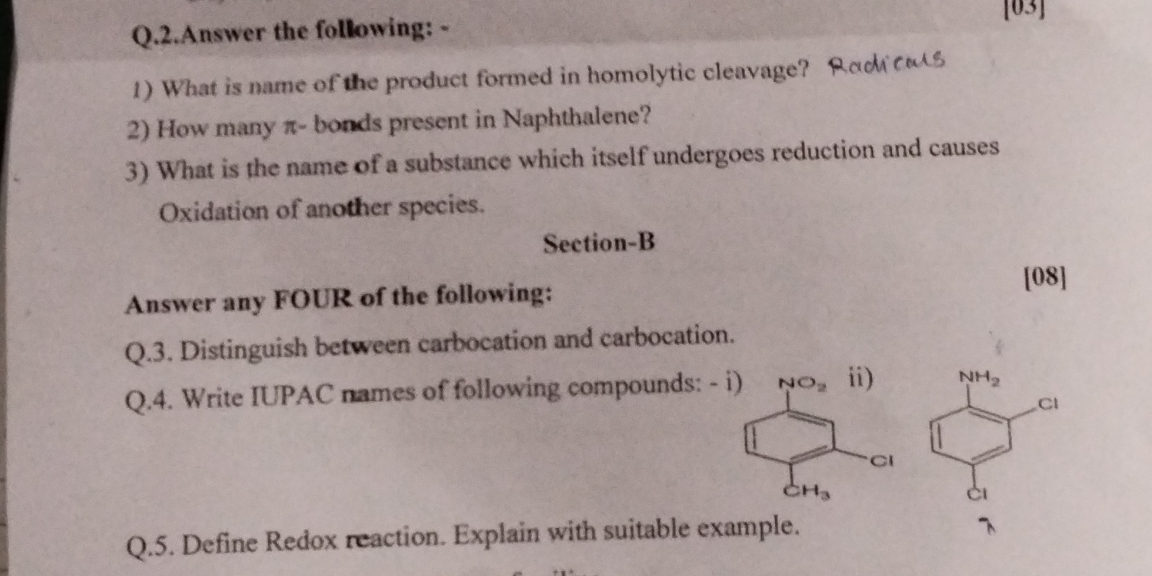
1) What is the name of the product formed in homolytic cleavage? 2) How many π-bonds are present in Naphthalene? 3) What is the name of a substance which itself undergoes reduction... 1) What is the name of the product formed in homolytic cleavage? 2) How many π-bonds are present in Naphthalene? 3) What is the name of a substance which itself undergoes reduction and causes oxidation of another species? 3) Distinguish between carbocation and carbocation. 4) Write IUPAC names of the following compounds: i) ii) Define Redox reaction. Explain with a suitable example.
Understand the Problem
The question consists of multiple parts relating to organic chemistry concepts, including the product of homolytic cleavage, the number of π-bonds in naphthalene, a substance that undergoes oxidation and reduction, and distinguishing between carbocations. It also includes tasks to write IUPAC names and define a Redox reaction.
Answer
1. Radicals, 2. Five π-bonds, 3. Oxidizing agent, 4. A positively charged carbocation vs negatively charged carbanion, 5. IUPAC: i) 3-Chloro-1-methyl-4-nitrobenzene, ii) 2,6-Dichloroaniline, 6. Redox reaction: electron transfer.
- Radicals
- Five π-bonds
- Oxidizing agent
- A carbocation is a positively charged carbon, while a carbanion is a negatively charged carbon.
- IUPAC names: i) 3-Chloro-1-methyl-4-nitrobenzene, ii) 2,6-Dichloroaniline.
- Redox reactions involve the transfer of electrons between two species, one being oxidized and the other reduced. Example: Zn + Cu^2+ -> Zn^2+ + Cu.
Answer for screen readers
- Radicals
- Five π-bonds
- Oxidizing agent
- A carbocation is a positively charged carbon, while a carbanion is a negatively charged carbon.
- IUPAC names: i) 3-Chloro-1-methyl-4-nitrobenzene, ii) 2,6-Dichloroaniline.
- Redox reactions involve the transfer of electrons between two species, one being oxidized and the other reduced. Example: Zn + Cu^2+ -> Zn^2+ + Cu.
More Information
Radicals are highly reactive species with unpaired electrons. Naphthalene, an aromatic hydrocarbon, contains five π-bonds across its two fused benzene rings. Oxidizing agents are substances that cause other species to lose electrons while they themselves gain electrons.
Tips
Confusing carbanion with carbocation due to similar naming. Ensure proper structure recognition for IUPAC naming.
Sources
- Homolytic & Heterolytic Fission of Covalent Bonds - BYJU'S - byjus.com
- Rules for Aromaticity: The 4 Key Factors - Master Organic Chemistry - masterorganicchemistry.com
- 9.1 Homolytic and Heterolytic Cleavage – Organic Chemistry I - kpu.pressbooks.pub
AI-generated content may contain errors. Please verify critical information