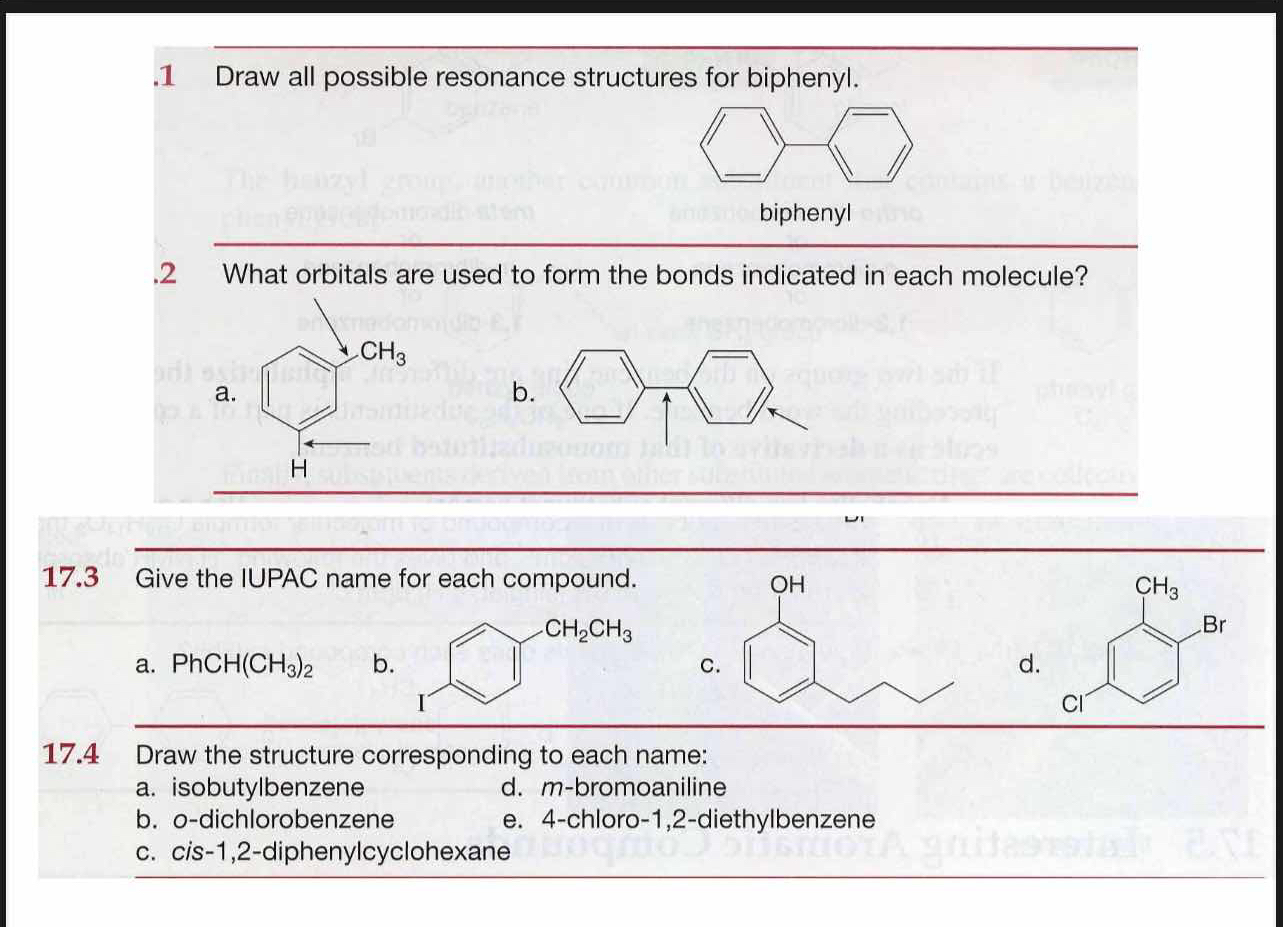
1. Draw all possible resonance structures for biphenyl. 2. What orbitals are used to form the bonds indicated in each molecule? 17.3 Give the IUPAC name for each compound. 17.4 Dra... 1. Draw all possible resonance structures for biphenyl. 2. What orbitals are used to form the bonds indicated in each molecule? 17.3 Give the IUPAC name for each compound. 17.4 Draw the structure corresponding to each name: a. isobutylbenzene b. o-dichlorobenzene c. cis-1,2-diphenylcyclohexane d. m-bromoaniline e. 4-chloro-1,2-diethylbenzene.
Understand the Problem
The questions are asking for resonance structures for biphenyl, the orbitals involved in bonds within specific molecules, to provide IUPAC names for given compounds, and to draw structures corresponding to certain names. This addresses multiple topics in organic chemistry, specifically relating to resonance, hybridization, nomenclature, and structural representation.
Answer
1. Alternating resonance in biphenyl. 2. σ: sp2, π: p orbitals. 3. IUPAC: a) 2-methyl-1-phenylpropane, b) 1-ethyl-4-iodobenzene, c) 4-(2-hydroxypropyl)phenol. 4. Draw respective structures.
- Biphenyl has alternating π bonds, resonating between each phenyl group. 2. Both a) and b) use sp2 orbitals for σ-bonds and p orbitals for π-bonds. 3. a) 2-methyl-1-phenylpropane, b) 1-ethyl-4-iodobenzene, c) 4-(2-hydroxypropyl)phenol. 4. Draw structures: a) isobutylbenzene, b) ortho-dichlorobenzene, c) cis-1,2-diphenylcyclohexane, d) meta-bromoaniline, e) 4-chloro-1,2-diethylbenzene.
Answer for screen readers
- Biphenyl has alternating π bonds, resonating between each phenyl group. 2. Both a) and b) use sp2 orbitals for σ-bonds and p orbitals for π-bonds. 3. a) 2-methyl-1-phenylpropane, b) 1-ethyl-4-iodobenzene, c) 4-(2-hydroxypropyl)phenol. 4. Draw structures: a) isobutylbenzene, b) ortho-dichlorobenzene, c) cis-1,2-diphenylcyclohexane, d) meta-bromoaniline, e) 4-chloro-1,2-diethylbenzene.
More Information
Biphenyl has conjugated π systems, allowing resonance. Aromatic compounds like benzene have sp2 hybridization and delocalized π-electrons, enabling stability and resonance properties in compounds like biphenyl.
Tips
It's common to forget that biphenyl involves resonating π bonds across the entire structure. Remember to consider hybridized orbitals when identifying bonds.
AI-generated content may contain errors. Please verify critical information