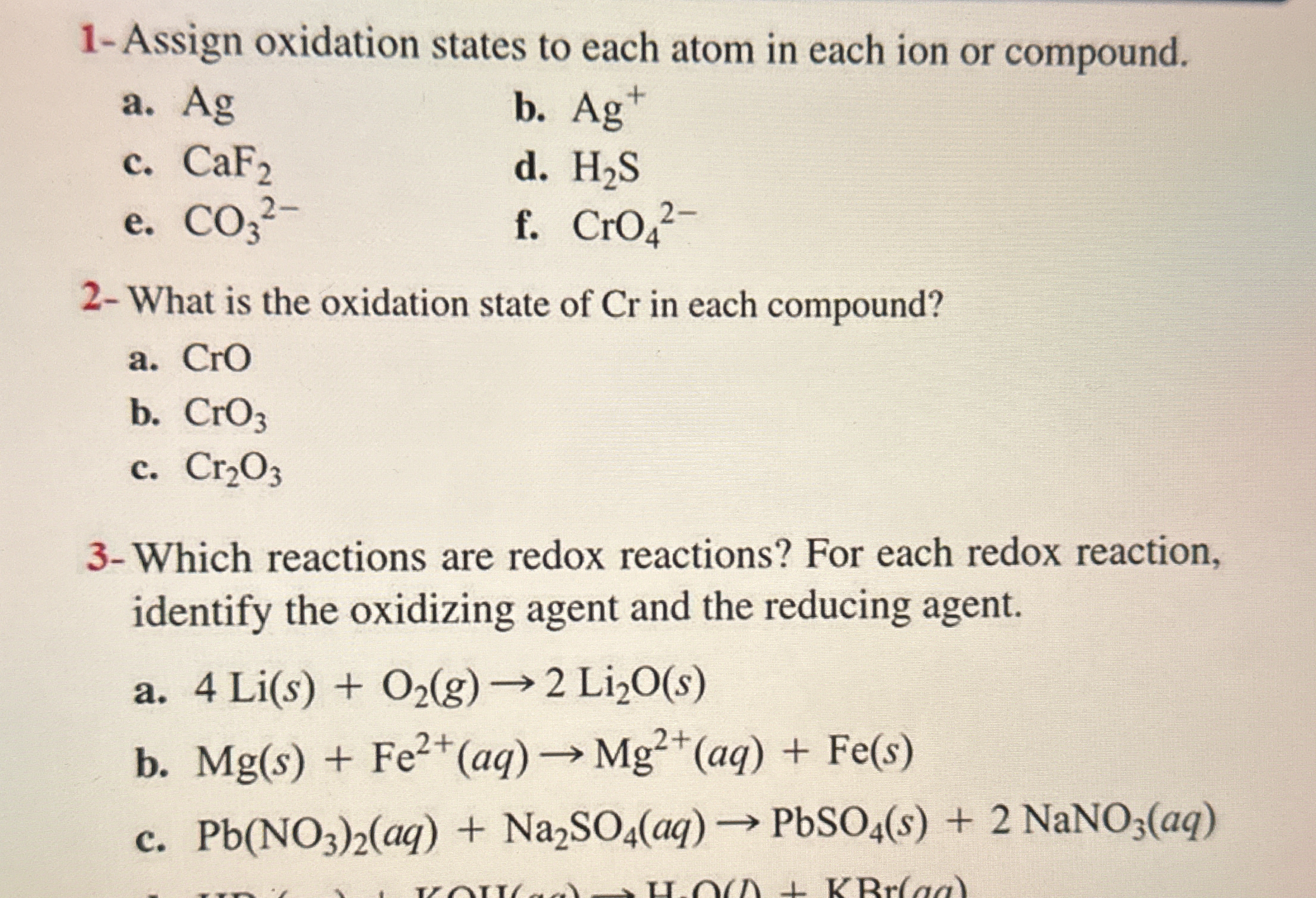
1- Assign oxidation states to each atom in each ion or compound. a. Ag b. Ag+ c. CaF2 d. H2S e. CO32- f. CrO42- 2- What is the oxidation state of Cr in each compound? a. CrO b. CrO... 1- Assign oxidation states to each atom in each ion or compound. a. Ag b. Ag+ c. CaF2 d. H2S e. CO32- f. CrO42- 2- What is the oxidation state of Cr in each compound? a. CrO b. CrO3 c. Cr2O3 3- Which reactions are redox reactions? For each redox reaction, identify the oxidizing agent and the reducing agent. a. 4 Li(s) + O2(g) → 2 Li2O(s) b. Mg(s) + Fe2+(aq) → Mg2+(aq) + Fe(s) c. Pb(NO3)2(aq) + Na2SO4(aq) → PbSO4(s) + 2 NaNO3(aq)
Understand the Problem
The questions ask for the determination of oxidation states in various compounds and ions, identification of oxidation states of chromium in specified compounds, and recognition of redox reactions along with the identification of oxidizing and reducing agents.
Answer
1a) Ag: 0, b) Ag+: +1, c) Ca+2, F-1, d) H+1, S-2, e) C+4, O-2, f) Cr+6, O-2. 2a) +2, b) +6, c) +3. 3a and b are redox, c is not.
- Oxidation states: a. Ag: 0, b. Ag+: +1, c. CaF2: Ca +2, F -1, d. H2S: H +1, S -2, e. CO3^2-: C +4, O -2, f. CrO4^2-: Cr +6, O -2. 2) Oxidation states of Cr: a. CrO: +2, b. CrO3: +6, c. Cr2O3: +3. 3) Redox reactions: a. Yes, oxidizing agent O2, reducing agent Li. b. Yes, oxidizing agent Fe2+, reducing agent Mg. c. No redox reaction.
Answer for screen readers
- Oxidation states: a. Ag: 0, b. Ag+: +1, c. CaF2: Ca +2, F -1, d. H2S: H +1, S -2, e. CO3^2-: C +4, O -2, f. CrO4^2-: Cr +6, O -2. 2) Oxidation states of Cr: a. CrO: +2, b. CrO3: +6, c. Cr2O3: +3. 3) Redox reactions: a. Yes, oxidizing agent O2, reducing agent Li. b. Yes, oxidizing agent Fe2+, reducing agent Mg. c. No redox reaction.
More Information
Redox reactions involve the transfer of electrons, leading to changes in oxidation states. Identifying these changes helps determine the oxidizing and reducing agents.
Tips
Common mistake: assuming a neutral compound has no overall charge, when actually the sum of oxidation numbers should equal zero.
Sources
AI-generated content may contain errors. Please verify critical information