Podcast
Questions and Answers
Which of the following is NOT a function of the kidney?
Which of the following is NOT a function of the kidney?
- Regulation of blood sugar levels
- Production of hormones
- Regulation of body fluid volume and osmolality
- Storage of dietary nutrients (correct)
What is the site where the renal artery, nerves, and renal vein enter/exit the kidney?
What is the site where the renal artery, nerves, and renal vein enter/exit the kidney?
- Renal Cortex
- Renal Papilla
- Renal Medulla
- Hilum (correct)
What is the name of the process by which the kidney synthesizes glucose from amino acids?
What is the name of the process by which the kidney synthesizes glucose from amino acids?
- Electrolyte regulation
- Glycolysis
- Gluconeogenesis (correct)
- Glycogenesis
What is the name of the hormone produced by the kidney that stimulates red blood cell production?
What is the name of the hormone produced by the kidney that stimulates red blood cell production?
What is the purpose of the renal papilla?
What is the purpose of the renal papilla?
What is the region between the renal pyramids composed of?
What is the region between the renal pyramids composed of?
What percentage of cardiac output does the kidney receive?
What percentage of cardiac output does the kidney receive?
What is the order of blood flow through the kidney?
What is the order of blood flow through the kidney?
What is the function of peritubular capillaries?
What is the function of peritubular capillaries?
How many nephrons are present in each kidney?
How many nephrons are present in each kidney?
What happens if nephrons are lost?
What happens if nephrons are lost?
What is the primary function of tubular secretion in the kidney?
What is the primary function of tubular secretion in the kidney?
What is the primary cause of chronic kidney disease?
What is the primary cause of chronic kidney disease?
What is the main difference between acute kidney injury and chronic kidney disease?
What is the main difference between acute kidney injury and chronic kidney disease?
What is the primary cause of post-renal acute kidney injury?
What is the primary cause of post-renal acute kidney injury?
What is the term for the final stage of chronic kidney disease, where dialysis is necessary?
What is the term for the final stage of chronic kidney disease, where dialysis is necessary?
What is the primary mechanism by which NSAIDs can cause acute kidney injury?
What is the primary mechanism by which NSAIDs can cause acute kidney injury?
What is the term for the classification of kidney disease based on the location of the damage?
What is the term for the classification of kidney disease based on the location of the damage?
What is the primary mechanism by which inadequate renal blood flow can lead to acute kidney injury?
What is the primary mechanism by which inadequate renal blood flow can lead to acute kidney injury?
What happens to the renal blood flow and GFR when there is high arterial blood pressure?
What happens to the renal blood flow and GFR when there is high arterial blood pressure?
What is the result of vasoconstriction of the efferent arteriole?
What is the result of vasoconstriction of the efferent arteriole?
What is the purpose of using creatinine in measuring GFR?
What is the purpose of using creatinine in measuring GFR?
What is the significance of 'transport maximum' in tubular reabsorption?
What is the significance of 'transport maximum' in tubular reabsorption?
What is the result of dilation of the afferent arteriole?
What is the result of dilation of the afferent arteriole?
Why do patients with severe hyperglycemia have glucose in their urine?
Why do patients with severe hyperglycemia have glucose in their urine?
What is the order of the tubular pathway?
What is the order of the tubular pathway?
What is the result of constriction of the afferent arteriole?
What is the result of constriction of the afferent arteriole?
What is the significance of macula densa in the regulation of GFR?
What is the significance of macula densa in the regulation of GFR?
What is the effect of dilation of the efferent arteriole on GFR?
What is the effect of dilation of the efferent arteriole on GFR?
What is the primary function of the glomerular capillaries?
What is the primary function of the glomerular capillaries?
What is the main difference between cortical and juxtamedullary nephrons?
What is the main difference between cortical and juxtamedullary nephrons?
What is the function of the visceral epithelium in the glomerulus?
What is the function of the visceral epithelium in the glomerulus?
What is the purpose of the juxtaglomerular apparatus?
What is the purpose of the juxtaglomerular apparatus?
What is the significance of the presence of large proteins like albumin in a urinalysis?
What is the significance of the presence of large proteins like albumin in a urinalysis?
What is the main function of the peritubular capillaries?
What is the main function of the peritubular capillaries?
What is the role of the basement membrane in the glomerulus?
What is the role of the basement membrane in the glomerulus?
What is the primary driving force for filtration in the nephron?
What is the primary driving force for filtration in the nephron?
What type of pressure is responsible for keeping too much fluid from filtering out through a semipermeable membrane?
What type of pressure is responsible for keeping too much fluid from filtering out through a semipermeable membrane?
What is the amount of excretion determined by in the nephron?
What is the amount of excretion determined by in the nephron?
In advanced cirrhosis, what is the primary factor contributing to increased fluid filtration into Bowman's space?
In advanced cirrhosis, what is the primary factor contributing to increased fluid filtration into Bowman's space?
What is the function of the macula densa in the juxtaglomerular apparatus?
What is the function of the macula densa in the juxtaglomerular apparatus?
What is the purpose of the glomerular endothelium in the glomerulus?
What is the purpose of the glomerular endothelium in the glomerulus?
What is the normal glomerular filtration rate (GFR) in mL/min?
What is the normal glomerular filtration rate (GFR) in mL/min?
What is the primary mechanism by which the kidneys autoregulate renal blood flow and maintain a constant GFR?
What is the primary mechanism by which the kidneys autoregulate renal blood flow and maintain a constant GFR?
What is the effect of increased systemic blood pressure on GFR?
What is the effect of increased systemic blood pressure on GFR?
What is the primary function of oncotic pressure in the nephron?
What is the primary function of oncotic pressure in the nephron?
What is the effect of decreased systemic blood pressure on GFR?
What is the effect of decreased systemic blood pressure on GFR?
What is the primary mechanism by which the kidneys maintain a constant GFR despite changes in systemic blood pressure?
What is the primary mechanism by which the kidneys maintain a constant GFR despite changes in systemic blood pressure?
What is the primary determinant of the amount of filtration surface area available in the nephron?
What is the primary determinant of the amount of filtration surface area available in the nephron?
What is the primary mechanism by which hypertension leads to renal failure in hypertensive nephropathy?
What is the primary mechanism by which hypertension leads to renal failure in hypertensive nephropathy?
Which of the following is a complication of uncontrolled diabetes that contributes to the development of diabetic nephropathy?
Which of the following is a complication of uncontrolled diabetes that contributes to the development of diabetic nephropathy?
What is the typical percentage of renal function at which dialysis is usually needed?
What is the typical percentage of renal function at which dialysis is usually needed?
Which of the following is a characteristic of autosomal recessive polycystic kidney disease?
Which of the following is a characteristic of autosomal recessive polycystic kidney disease?
What is the primary function of the peritoneal membrane in peritoneal dialysis?
What is the primary function of the peritoneal membrane in peritoneal dialysis?
Which of the following is a common feature of autosomal dominant polycystic kidney disease?
Which of the following is a common feature of autosomal dominant polycystic kidney disease?
What is the primary mechanism by which the kidneys adapt to chronic insults and maintain normal renal function?
What is the primary mechanism by which the kidneys adapt to chronic insults and maintain normal renal function?
What is the primary site of damage in diabetic nephropathy?
What is the primary site of damage in diabetic nephropathy?
What is the primary advantage of peritoneal dialysis over hemodialysis?
What is the primary advantage of peritoneal dialysis over hemodialysis?
Which of the following is a common complication of autosomal recessive polycystic kidney disease?
Which of the following is a common complication of autosomal recessive polycystic kidney disease?
Which of the following is a characteristic of Nephritic Syndrome?
Which of the following is a characteristic of Nephritic Syndrome?
What is the primary mechanism of glomerular damage in Nephritic Syndrome?
What is the primary mechanism of glomerular damage in Nephritic Syndrome?
What is the primary cause of hypertension in Glomerular Disease?
What is the primary cause of hypertension in Glomerular Disease?
What is the primary distinguishing feature between Nephrotic and Nephritic Syndromes?
What is the primary distinguishing feature between Nephrotic and Nephritic Syndromes?
What is the primary consequence of glomerular damage leading to 'holes' in the glomerular basement membrane?
What is the primary consequence of glomerular damage leading to 'holes' in the glomerular basement membrane?
What is the primary factor that determines which syndrome a patient with glomerular damage will manifest?
What is the primary factor that determines which syndrome a patient with glomerular damage will manifest?
What percentage of patients with Minimal Change Glomerulonephritis recover completely?
What percentage of patients with Minimal Change Glomerulonephritis recover completely?
What is the primary mechanism by which the Juxtaglomerular Apparatus responds to low GFR?
What is the primary mechanism by which the Juxtaglomerular Apparatus responds to low GFR?
What is the term for the glomerular disease that is characterized by a sudden appearance of proteinuria and edema?
What is the term for the glomerular disease that is characterized by a sudden appearance of proteinuria and edema?
What is the primary cause of nephrotic syndrome?
What is the primary cause of nephrotic syndrome?
What is the primary function of the proximal tubule?
What is the primary function of the proximal tubule?
What is the characteristic feature of minimal change nephropathy?
What is the characteristic feature of minimal change nephropathy?
What is the term for the mechanism by which the Loop of Henle concentrates urine?
What is the term for the mechanism by which the Loop of Henle concentrates urine?
What is the term for the process by which tubular epithelial cells reabsorb substances from the filtrate?
What is the term for the process by which tubular epithelial cells reabsorb substances from the filtrate?
What is the primary difference between nephritic and nephrotic syndrome?
What is the primary difference between nephritic and nephrotic syndrome?
What is the primary mechanism of acute glomerulonephritis?
What is the primary mechanism of acute glomerulonephritis?
What is the term for the disease that is characterized by significant fibrous scar tissue, hypercellularity, and obliteration of the glomerular capillaries?
What is the term for the disease that is characterized by significant fibrous scar tissue, hypercellularity, and obliteration of the glomerular capillaries?
What is the primary characteristic of post-streptococcal glomerulonephritis?
What is the primary characteristic of post-streptococcal glomerulonephritis?
What is the most common cause of chronic renal failure?
What is the most common cause of chronic renal failure?
What is the term for the cells that connect the proximal tubule to the glomerulus?
What is the term for the cells that connect the proximal tubule to the glomerulus?
What is the primary difference between acute and chronic glomerulonephritis?
What is the primary difference between acute and chronic glomerulonephritis?
What is the primary function of the glomerulus?
What is the primary function of the glomerulus?
What is the term for the process by which the kidneys concentrate urine?
What is the term for the process by which the kidneys concentrate urine?
What is the term for the disease that is characterized by uniform and diffuse effacement of podocytes?
What is the term for the disease that is characterized by uniform and diffuse effacement of podocytes?
What is the primary mechanism of proteinuria in nephrotic syndrome?
What is the primary mechanism of proteinuria in nephrotic syndrome?
What is the primary cause of hyperlipidemia in nephrotic syndrome?
What is the primary cause of hyperlipidemia in nephrotic syndrome?
What is the typical duration between the original infection and the onset of symptoms in post-strep glomerulonephritis?
What is the typical duration between the original infection and the onset of symptoms in post-strep glomerulonephritis?
What is the characteristic pattern of antibody deposition in Goodpasture's syndrome?
What is the characteristic pattern of antibody deposition in Goodpasture's syndrome?
What is the primary mechanism of damage in Type II Rapidly Progressive Glomerulonephritis (RPGN)?
What is the primary mechanism of damage in Type II Rapidly Progressive Glomerulonephritis (RPGN)?
What is the characteristic feature of the glomerular injury in Membranous Glomerulonephritis?
What is the characteristic feature of the glomerular injury in Membranous Glomerulonephritis?
What is the most common type of Rapidly Progressive Glomerulonephritis (RPGN)?
What is the most common type of Rapidly Progressive Glomerulonephritis (RPGN)?
What is the typical rate of decline in glomerular filtration rate (GFR) in untreated Rapidly Progressive Glomerulonephritis (RPGN)?
What is the typical rate of decline in glomerular filtration rate (GFR) in untreated Rapidly Progressive Glomerulonephritis (RPGN)?
What is the hallmark of Goodpasture's syndrome?
What is the hallmark of Goodpasture's syndrome?
What is the typical presentation of Rapidly Progressive Glomerulonephritis (RPGN)?
What is the typical presentation of Rapidly Progressive Glomerulonephritis (RPGN)?
What is the primary function of the ascending limb in the loop of Henle?
What is the primary function of the ascending limb in the loop of Henle?
Which of the following is a function of anti-diuretic hormone (ADH)?
Which of the following is a function of anti-diuretic hormone (ADH)?
What is the effect of loop diuretics on the nephron?
What is the effect of loop diuretics on the nephron?
What is the primary function of aldosterone in the distal tubule?
What is the primary function of aldosterone in the distal tubule?
What is the effect of thiazide diuretics on the nephron?
What is the effect of thiazide diuretics on the nephron?
What is the primary cause of obstructive uropathy?
What is the primary cause of obstructive uropathy?
What is the primary function of the collecting duct?
What is the primary function of the collecting duct?
What is the effect of potassium-sparing diuretics on the nephron?
What is the effect of potassium-sparing diuretics on the nephron?
What is the primary function of the descending limb of the loop of Henle?
What is the primary function of the descending limb of the loop of Henle?
What is the result of constriction of the efferent arteriole?
What is the result of constriction of the efferent arteriole?
What triggers the growth of new tissue in the ureters in obstructive uropathy?
What triggers the growth of new tissue in the ureters in obstructive uropathy?
What is the primary cause of Acute Tubular Necrosis (ATN)?
What is the primary cause of Acute Tubular Necrosis (ATN)?
What is the result of elevated hydrostatic pressure in Bowman's Capsule?
What is the result of elevated hydrostatic pressure in Bowman's Capsule?
What is the timeline for glomerular damage in obstructive uropathy?
What is the timeline for glomerular damage in obstructive uropathy?
What is the effect of fibrosis in the tubules and interstitial space?
What is the effect of fibrosis in the tubules and interstitial space?
What is the common symptom of glomerular disease in obstructive uropathy?
What is the common symptom of glomerular disease in obstructive uropathy?
What is the primary cause of Ischemic Acute Tubular Necrosis (ATN)?
What is the primary cause of Ischemic Acute Tubular Necrosis (ATN)?
What is the hallmark of the Initiation Phase of ATN?
What is the hallmark of the Initiation Phase of ATN?
What is the most common indication for Hemodialysis in the acute setting of ATN?
What is the most common indication for Hemodialysis in the acute setting of ATN?
What is the primary reason why we care about ATN?
What is the primary reason why we care about ATN?
What is the result of ischemia in the tubular cells in ATN?
What is the result of ischemia in the tubular cells in ATN?
What is the characteristic appearance of urine in ATN?
What is the characteristic appearance of urine in ATN?
What is the primary mechanism of Nephrotoxic ATN?
What is the primary mechanism of Nephrotoxic ATN?
What is the final stage of the phases of ATN?
What is the final stage of the phases of ATN?
What is the primary function of aquaporins in the tubules?
What is the primary function of aquaporins in the tubules?
What is the main organ involved in osmoregulation, along with the kidneys?
What is the main organ involved in osmoregulation, along with the kidneys?
What is the primary mechanism by which vasopressin (ADH) regulates water reabsorption in the collecting ducts?
What is the primary mechanism by which vasopressin (ADH) regulates water reabsorption in the collecting ducts?
What is the normal range of plasma osmolality?
What is the normal range of plasma osmolality?
What is the primary function of the proximal tubules in the nephron?
What is the primary function of the proximal tubules in the nephron?
What is the effect of vasopressin (ADH) on the collecting ducts?
What is the effect of vasopressin (ADH) on the collecting ducts?
What is the primary function of the descending limb of the loop of Henle?
What is the primary function of the descending limb of the loop of Henle?
What is the primary effect of hypernatremia on the body's cells?
What is the primary effect of hypernatremia on the body's cells?
What is the term for the concentration of solutes in body fluids?
What is the term for the concentration of solutes in body fluids?
What is the most common cause of hypovolemic hypernatremia?
What is the most common cause of hypovolemic hypernatremia?
Why is phosphorus essential for normal body function?
Why is phosphorus essential for normal body function?
What is the term for the loss of free water leading to hypernatremia?
What is the term for the loss of free water leading to hypernatremia?
What is the consequence of rapid changes in plasma osmolality on the brain?
What is the consequence of rapid changes in plasma osmolality on the brain?
What is the primary mechanism by which sodium homeostasis is maintained?
What is the primary mechanism by which sodium homeostasis is maintained?
What is the direct effect of decreased body fluid osmolarity on vasopressin secretion?
What is the direct effect of decreased body fluid osmolarity on vasopressin secretion?
What is the primary mechanism by which decreased plasma volume leads to increased vasopressin secretion?
What is the primary mechanism by which decreased plasma volume leads to increased vasopressin secretion?
In diabetes insipidus, what is the primary defect that leads to increased water excretion?
In diabetes insipidus, what is the primary defect that leads to increased water excretion?
What is the primary consequence of SIADH (Syndrome of Inappropriate ADH) on plasma osmolarity?
What is the primary consequence of SIADH (Syndrome of Inappropriate ADH) on plasma osmolarity?
What is the mechanism by which sodium reabsorption in the kidney generates an electrochemical gradient?
What is the mechanism by which sodium reabsorption in the kidney generates an electrochemical gradient?
What is the primary function of the 'conveyor belt' mechanism in sodium reabsorption?
What is the primary function of the 'conveyor belt' mechanism in sodium reabsorption?
What is the effect of decreased vasopressin secretion on water reabsorption in the collecting ducts?
What is the effect of decreased vasopressin secretion on water reabsorption in the collecting ducts?
What is the primary cause of hypernatremia in diabetes insipidus?
What is the primary cause of hypernatremia in diabetes insipidus?
What is the primary consequence of SIADH on urine osmolarity?
What is the primary consequence of SIADH on urine osmolarity?
What is the primary mechanism by which the kidneys respond to changes in plasma osmolarity?
What is the primary mechanism by which the kidneys respond to changes in plasma osmolarity?
What is the primary function of the juxtaglomerular apparatus?
What is the primary function of the juxtaglomerular apparatus?
What happens when potassium levels are high in the adrenal gland?
What happens when potassium levels are high in the adrenal gland?
What is the primary mechanism of potassium regulation in the distal tubule and collecting duct?
What is the primary mechanism of potassium regulation in the distal tubule and collecting duct?
What is the main source of potassium gain in the body?
What is the main source of potassium gain in the body?
What is the effect of massive cellular breakdown on potassium levels?
What is the effect of massive cellular breakdown on potassium levels?
What is the role of the macula densa in the juxtaglomerular apparatus?
What is the role of the macula densa in the juxtaglomerular apparatus?
What is the primary determinant of resting membrane potential?
What is the primary determinant of resting membrane potential?
What is the function of Na+/K+ ATPase in potassium regulation?
What is the function of Na+/K+ ATPase in potassium regulation?
What is the primary cause of hyperkalemia in a patient with acute renal failure?
What is the primary cause of hyperkalemia in a patient with acute renal failure?
What is the effect of acidic pH on potassium levels?
What is the effect of acidic pH on potassium levels?
What is the primary mechanism of potassium removal in the treatment of hyperkalemia?
What is the primary mechanism of potassium removal in the treatment of hyperkalemia?
What is the effect of thiazide diuretics on potassium levels?
What is the effect of thiazide diuretics on potassium levels?
What is the primary cause of hypokalemia in a patient with diarrhea?
What is the primary cause of hypokalemia in a patient with diarrhea?
What is the effect of insulin on potassium levels?
What is the effect of insulin on potassium levels?
What is the primary mechanism of action of calcium gluconate in the treatment of hyperkalemia?
What is the primary mechanism of action of calcium gluconate in the treatment of hyperkalemia?
What is the primary cause of hyperkalemia in a patient with hypoaldosteronism?
What is the primary cause of hyperkalemia in a patient with hypoaldosteronism?
What is the effect of alkalosis on potassium levels?
What is the effect of alkalosis on potassium levels?
What is the primary mechanism of action of IV sodium bicarbonate in the treatment of hyperkalemia?
What is the primary mechanism of action of IV sodium bicarbonate in the treatment of hyperkalemia?
What is the primary mechanism by which the kidneys regulate serum pH?
What is the primary mechanism by which the kidneys regulate serum pH?
What happens to the bicarbonate ions in the tubule during renal compensation for acidosis?
What happens to the bicarbonate ions in the tubule during renal compensation for acidosis?
What is the effect of hyperventilation on serum pH?
What is the effect of hyperventilation on serum pH?
What is the primary source of H+ ions in the body?
What is the primary source of H+ ions in the body?
What is the role of the kidneys in maintaining acid-base homeostasis?
What is the role of the kidneys in maintaining acid-base homeostasis?
What is the effect of alkalosis on the body?
What is the effect of alkalosis on the body?
What is the role of the lungs in maintaining acid-base homeostasis?
What is the role of the lungs in maintaining acid-base homeostasis?
What is the effect of respiratory acidosis on the body?
What is the effect of respiratory acidosis on the body?
Study Notes
The Renal System
- The kidney is the main organ responsible for maintaining homeostasis, controlling the composition of body fluids and excretion of metabolic waste and foreign substances.
- The kidney regulates body fluid volume, osmolality, and acid-base balance, and also produces hormones such as erythropoietin and activates vitamin D.
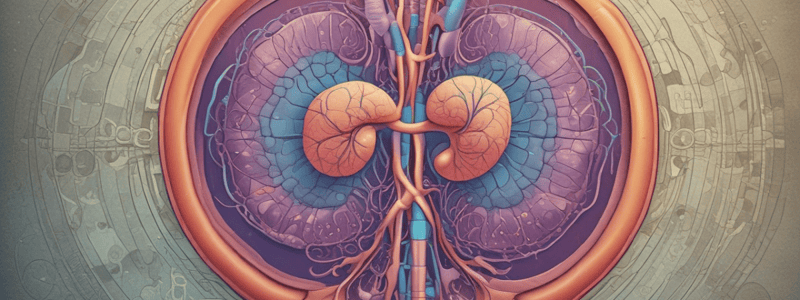
Gross Anatomy of the Kidney
- The kidney is a retroperitoneal organ located alongside the vertebral column at T12-L3.
- The kidney is divided into the outer cortex and inner medulla, with the hilum being the point where the renal artery and nerves enter and the renal vein exits.
- The ureter exits the kidney to drain urine into the bladder.
Renal Perfusion
- The kidney is one of the most well-perfused organs in the body, receiving 25% of cardiac output.
- The right and left renal arteries branch off the abdominal aorta and enter the kidney through the hilum.
- The blood flow through the kidney is divided into two capillary beds: glomerular and peritubular.
The Nephron
- The nephron is the functional unit of the kidney, with approximately 1 million nephrons per kidney.
- The nephron is divided into the glomerulus, proximal tubule, loop of Henle, and distal tubule.
- The nephron is responsible for filtering plasma, reabsorbing and secreting substances, and regulating the amount of substance excreted in the urine.
Glomerular Filtration
- Glomerular filtration is a passive process that occurs through the glomerular capillaries, driven by hydrostatic and osmotic pressures.
- The filtration rate is influenced by the surface area available for filtration, membrane permeability, and net filtration pressure.
- The glomerular filtration rate (GFR) is the volume of plasma filtered per unit time, with a normal rate of 125 mL/min.
Autoregulation of Renal Blood Flow
- The kidney can autoregulate blood flow in response to changes in systemic blood pressure, maintaining a constant GFR.
- Autoregulation occurs through two mechanisms: myogenic autoregulation and feedback from the juxtaglomerular apparatus.
Regulation of Renal Blood Flow and GFR
- The kidney can regulate GFR by adjusting the diameter of the afferent and efferent arterioles.
- The kidney can increase GFR by dilating the afferent arteriole and constricting the efferent arteriole, and decrease GFR by constricting the afferent arteriole and dilating the efferent arteriole.
Clinical Correlations
- Estimating GFR is an important indicator of kidney function.
- Creatinine is a commonly used substance to estimate GFR, as it is freely filtered in the glomerulus but not reabsorbed in the tubules.
- GFR can be influenced by age, gender, and muscle mass.
Tubular Pathway Review
- The tubular pathway consists of the proximal tubule, loop of Henle, and distal convoluted tubule.
- Transport (reabsorption and secretion) occurs in every part of the tubule.
- The proximal tubule is responsible for reabsorbing most of the filtered sodium, water, and other solutes.
Tubular Reabsorption
- Tubular reabsorption is the process of transporting substances from the tubular lumen back into the peritubular capillaries.
- Transport can be active or passive.
- The tubules have a transport maximum, beyond which they cannot reabsorb any more substances.
Tubular Secretion
- Tubular secretion is the process of transporting substances from the peritubular capillaries into the tubular lumen.
- Important for disposing of drugs and drug metabolites, eliminating undesirable substances, removing excess potassium, and controlling pH.
Pathophysiology of the Kidney
- Each function of the kidney can be compromised, leading to various disorders.
- The result of the compromised function will depend on the location, etiology, and chronicity of the damage.
Classifying Renal Disease
- Renal disease can be classified based on chronicity, location, and etiology.
- Acute kidney injury (AKI) and chronic kidney disease (CKD) are two common classifications.
Causes of Kidney Injury
- Prerenal causes: inadequate renal blood flow, leading to decreased GFR.
- Intrarenal causes: nephron damage, glomerulotubular diseases.
- Postrenal causes: obstruction or structural defects blocking urine from draining.
Chronic Kidney Disease
- Slow, progressive loss of renal function and decrease in GFR.
- Causes: systemic diseases, intrinsic kidney disease, and obstructive uropathies.
- Defined in stages based on GFR and whether the patient is dialysis-dependent.
End-Stage Renal Disease
- Dialysis is needed to sustain life.
- Can be caused by various diseases, including hypertension, diabetes, and autoimmune conditions.
Hypertensive Nephropathy
- Can affect both glomerular and peritubular capillaries.
- Microvascular damage from constant exposure to high systemic blood pressure.
- Leads to tissue fibrosis, ischemia, and small/fibrotic glomeruli.
Diabetic Nephropathy
- Hyperglycemia over time causes microvascular damage to renal arteries and direct glomerular damage.
- Leads to decreased GFR, proteinuria, and eventually end-stage renal disease.
CKD and Renal Adaptation
- The kidney has a remarkable ability to adapt to chronic insults, allowing for preservation of renal function.
- However, eventually, the kidneys can no longer compensate, and dialysis may be needed.
Dialysis
- Hemodialysis and peritoneal dialysis are two common methods of dialysis.
- Hemodialysis uses an external glomerular membrane and tubule system to filter undesired substances, remove volume, and maintain a normal balance of solutes.
- Peritoneal dialysis uses the peritoneal membrane to filter plasma and remove waste products.
Glomerular Diseases
- Impairment of glomerular filtering properties leads to decreased GFR
- Can be primary or secondary
- Molecules like blood cells and Albumin can pass into urine and be excreted
Nephrotic and Nephritic Syndromes
- Syndromes are constellations of signs and symptoms with multiple causes
- Depend on amount and type of damage
- Not all glomerular damage causes syndromes, and some diseases can cause both
Nephritic Syndrome
- Inflammatory cells accumulate in the mesangium
- Caused by antigen deposition, leading to damage to endothelial cells
- Damage thins the glomerular basement membrane and podocytes, allowing proteins and red cells through into the filtrate
- Signs: Hematuria, Hypertension, Oliguria, Edema, RBC casts and red blood cells, Protein (to a lesser degree), White blood cells
- Associated with: Infection-related Glomerulonephritis, Rapidly Progressive Glomerulonephritis (RPGN)
Nephrotic Syndrome
- Damage to the glomerular basement membrane/podocytes leads to loss of negative charge and increased permeability
- Plasma proteins are lost, decreasing plasma oncotic pressure
- Can be primary or secondary
- Diabetic nephropathy is the most common cause of secondary, HTN is the second most common
- Signs: Proteinuria (>3.5 gm/day), Hypoalbuminemia, Ascites and peripheral edema, Hyperlipidemia, Lipiduria
Glomerulopathy
- Disease of the glomeruli
- Group of autoimmune disorders involving inflammation of glomerular capillaries
- Present with various combinations of hematuria, proteinuria, oliguria, hypertension
Types of Glomerulonephritis
- Acute Glomerulonephritis: Primary glomerular injury from immunologic responses, ischemia, drugs, toxins, infection
- Post-Streptococcal Glomerulonephritis: One of the causes of Acute Glomerulonephritis
- Rapidly Progressive Glomerulonephritis (RPGN): Autoimmune, can be secondary to post-strep GN or unknown causes, always autoimmune
- Membranous Glomerulonephritis: One of the most common causes of Nephrotic syndrome in adults
- Minimal Change Glomerulonephritis: One of the most common causes of Nephrotic syndrome in children
- Chronic Glomerulonephritis: Slow progression, can be asymptomatic, often an incidental finding on a routine physical
- Secondary Glomerulonephritis: Most common cause of chronic renal failure, often due to DM, HTN, or both
Path of Damage in Obstructive Uropathy
- Collecting system dilates, triggering smooth muscle hypertrophy in the ureters
- Flow slows down or stops, decreasing GFR and elevating hydrostatic pressure in Bowman's Capsule
- Collecting system stretches and enlarges, damaging tubular cells and leading to fibrosis
- Fibrosis signals tissue repair, bringing in cytokines, proteases, and causing cell damage and apoptosis
- Irreversible tubular damage can result
General Timeline
- < 1 week: Hydronephrosis and hydroureter are present
- ~ 1 week: Distal tubules show evidence of damage
- ~ 2 weeks: Proximal tubule damage
- ~ 4 weeks: Glomeruli are damaged
- > 4 weeks: Cell death begins to occur
- > 3 months: Doubtful that any function can be regained
Clinical Correlation: Signs/Symptoms
- Flank pain
- Fever
- Nausea/vomiting
- Hematuria (especially in intrinsic causes like stones)
- Symptoms of glomerular disease like edema if the obstruction persists
Acute Tubular Necrosis (ATN)
Ischemic ATN
- Hypoperfusion leads to hypoxia, causing cell death
- Cellular debris of dead cells (casts) fall into the tubules, causing physical obstruction
- Obstruction lowers GFR more due to initial hypoperfusion
- Ischemia in tubular cells stops production of vasodilators, leading to more vasoconstriction
- Tubules are affected in an interrupted and "patchy" way
- Debris from dead tubular cells can be seen as granular "casts" in the urine, which looks "muddy"
Etiology of Ischemic ATN
- Renal hypoperfusion
- Tubule cell injury
- Tubule cell death
- Cellular debris → Tubular occlusion
- Lowered GFR
- More vasoconstriction
Nephrotoxic ATN
- Occurs due to exposure to toxic agents
- Nephrotoxic drugs (e.g., antibiotics, chemotherapy, contrast dye)
- Bacterial toxins
- Large amounts of circulating hemoglobin or myoglobin
- Toxic non-drug substances (e.g., antifreeze, mercury, arsenic)
Phases of ATN
Initiation Phase
- Drastic decrease in GFR
- Big "jump" in BUN/creatinine levels
- Clinically, suspect ATN if creatinine rises sharply in the first 24-48 hours
Maintenance Phase
- GFR remains low
- BUN/Cr continue to rise
- Oliguria or anuria
- Hemodialysis may become necessary
Clinical Correlation: Indications for HD in the Acute Setting
- Volume overload
- Hyperkalemia
- Severe acid-base disturbances
- Uremic encephalopathy
- Toxin/medication removal
Recovery Phase
- Tubule cells begin regenerating
- Tubular function can slowly recover
- First indication is usually increasing urine output and then gradual decrease in BUN/Cr
- Similar to obstruction, there can be "post-ATN diuresis" due to dilute urine from regenerating tubular cells
- Recovery can be complete, partial, or never, and some patients end up with life-long ESRD and remain dialysis-dependent
Clinical Correlation: Why should we care about ATN?
- 22.7% incidence of AKI in hospitalizations
- AKI is associated with a more than fourfold increased likelihood of death
- ATN is the most common cause of AKI
Body Fluid Compartments
- Total body fluid is divided into ⅔ intracellular fluid (fluid within cells) and ⅓ extracellular fluid
- Extracellular fluid is further divided into ¾ interstitial fluid (between cells) and ¼ plasma fluid (in capillaries)
- Plasma fluid is responsible for transporting materials between cells and capillaries through interstitial fluid
Osmoregulation
- Osmolality is the concentration of solutes in body fluids, critically important for organ function
- Normal plasma osmolality is maintained by:
- Excreting excess water
- Replenishing lost water
- Maintaining normal amounts of solutes in the body
- The brain and kidneys are the main organs involved in osmoregulation
Maintaining Body Water
- Water reabsorption occurs passively through osmosis, but is ultimately determined by the movement of sodium and the presence of aquaporins
- Sodium is the most abundant extracellular cation
- Aquaporins are surface proteins that form "holes" in the cellular membrane, allowing water to pass through
- Aquaporins are present throughout the tubules, with high concentrations in the proximal tubules and descending limb of the loop of Henle
- No aquaporins are present in the collecting ducts unless vasopressin (ADH) is present, making water reabsorption dependent on ADH
Vasopressin/ADH Control of Water Reabsorption
- Vasopressin is released by the posterior pituitary in response to elevated osmolarity and stimulates water reabsorption in the collecting ducts
- Decreased osmolarity inhibits vasopressin secretion, leading to decreased water reabsorption
- Baroreceptors in the atria and carotid arteries also affect vasopressin production, with low blood pressure stimulating release of vasopressin
Disorders of Vasopressin
- Diabetes Insipidus (DI):
- Central DI: brain produces too little ADH, often due to pituitary or hypothalamic damage
- Nephrogenic DI: kidneys don't respond to circulating ADH, often due to chronic lithium use or hypercalcemia
- SIADH (Syndrome of Inappropriate ADH):
- Body is unable to suppress ADH secretion, leading to excessive water reabsorption and hyponatremia
- Caused by CNS issues, malignancy, or certain medications
Sodium Homeostasis
- Sodium reabsorption from tubule cells to interstitial fluid is almost always active transport
- Active transport generates an electrochemical gradient, allowing for the reabsorption of other substances like glucose
- Na+/K+ ATPase is the enzyme responsible for active sodium reabsorption
Sodium Reabsorption
- Sodium is actively pumped out of the tubule and back into the blood, allowing other substances to be reabsorbed
- Energy is expended to "pull" sodium from the tubular lumen into the cell, creating an electrochemical gradient
- Sodium is then actively transported out of the cell to maintain low intracellular Na+ concentrations
Disorders of Sodium Regulation
- Hypernatremia: too much sodium (Na >145 mg/dL), causing fluid to shift from intracellular fluid to extracellular fluid
- Causes of hypernatremia:
- Hypovolemic hypernatremia: dehydration, osmotic diuresis
- Hypervolemic hypernatremia: excessive sodium intake, iatrogenic administration of normal saline
- Diabetes Insipidus: loss of free water
- Hyponatremia: too little sodium (Na <135 mg/dL), often caused by SIADH, heart failure, or liver disease
Juxtaglomerular Apparatus
- JG cells around the afferent arteriole are specialized smooth muscle cells that secrete renin and control blood flow into the glomerulus.
- Mechanoreceptors in JG cells sense changes in the vessel diameter, and when blood pressure is low, they release more renin.
- Macula densa, located in the distal convoluted tubule, senses changes in sodium concentration of the filtrate and signals to the JG cells to release more renin when sodium levels are low.
Potassium Regulation
- Potassium is the major intracellular cation, and its distribution is maintained by Na+/K+ ATPase pumps.
- K+ sensors in the adrenal gland respond to changes in potassium levels, and high potassium levels stimulate the release of aldosterone, which promotes potassium excretion and sodium retention.
- Low potassium levels lead to decreased aldosterone release, resulting in potassium retention and sodium excretion.
- Normal intracellular potassium levels are crucial for maintaining resting membrane potential, and changes in potassium levels can affect muscle contraction and neuronal activity.
Renal Regulation of Potassium
- Bulk reabsorption of potassium occurs in the proximal tubule and the loop of Henle.
- The distal tubule and collecting duct are responsible for regulating potassium levels in response to aldosterone.
- Aldosterone stimulates the production of proteins that activate the Na+/K+ ATPase, leading to sodium reabsorption and potassium secretion.
Factors Affecting Plasma K+
- Factors that increase plasma potassium levels include:
- Dietary intake
- Cellular breakdown
- Acidosis
- Factors that decrease plasma potassium levels include:
- Stool losses
- Increased cellular uptake
- Insulin and acid-base status
Potassium and Serum pH
- Potassium is an important buffer in the intracellular fluid.
- When there is high plasma H+ (acidosis), H+ ions move into the cell, and potassium ions move out of the cell, leading to increased plasma potassium levels.
- Alternatively, when there is high plasma pH (alkalosis), H+ ions move out of the cell, and potassium ions move into the cell, leading to decreased plasma potassium levels.
Pathophysiology of Hyperkalemia
- Hyperkalemia is typically defined as plasma potassium levels > 5.5 mmol/L.
- Causes of hyperkalemia include:
- Decreased renal excretion due to decreased filtration or secretion
- Acute renal failure
- Hypoaldosteronism
- Medication effects
- Cellular breakdown
- Clinical correlations:
- Treatment of hyperkalemia includes calcium gluconate, insulin + D50, Kayexalate, and IV sodium bicarbonate.
- Patients with end-stage renal disease on hemodialysis must be mindful of dietary potassium intake.
Pathophysiology of Hypokalemia
- Hypokalemia is not as life-threatening as hyperkalemia.
- Causes of hypokalemia include:
- Excess urinary losses
- Excess stool losses
- Increased cellular uptake
- Alkalosis
- Pregnancy
- Treatment of hypokalemia includes potassium replacement, either orally or intravenously.
Renal Acid-Base Buffering
- Acids tend to give up hydrogen ions, while bases combine with hydrogen ions to neutralize them.
- The shape of a protein determines its action, and protein shape is dependent on pH.
- Derangements in serum pH can lead to significant dysfunction throughout the body and ultimately death.
Hydrogen Ion Buffering
- The lungs and kidneys work together to maintain a balance of H+ and HCO3 ions to maintain normal pH.
- Excess hydrogen in the blood is buffered by bicarbonate, which combines with hydrogen to form carbonic acid.
- Not enough hydrogen in the blood leads to the dissociation of carbonic acid into hydrogen and bicarbonate ions.
Acidosis and Alkalosis
- Acidosis pushes the equation towards the left, forming more carbonic acid, while alkalosis pushes it towards the right, promoting the dissociation of carbonic acid into hydrogen and bicarbonate ions.
- Respiratory compensation occurs quickly, while renal buffering takes more time.
- The kidneys and lungs work together continuously to regulate serum H+ concentrations.
Bicarbonate Reabsorption
- Bicarbonate is freely filtered in the glomerulus and must be reabsorbed in the tubule.
- The majority of bicarbonate reabsorption occurs in the proximal tubule.
- Within a tubule cell, water and CO2 combine to form carbonic acid, which dissociates into bicarbonate and hydrogen ions.
- H+ moves into the tubular lumen, and bicarbonate moves out of the cell and back into the plasma.
Renal pH Control
- To increase serum pH, the kidneys reabsorb more bicarbonate.
- To decrease serum pH, the kidneys excrete more bicarbonate.
Renal Compensation for Acidosis
- If the plasma is too acidic, the kidneys reabsorb more bicarbonate, but the bloodstream still wants more.
- This leads to the renal compensation for chronic respiratory acidosis.
- Within a tubule cell, water and CO2 come together to form bicarbonate, which is reabsorbed.
- H+ moves into the tubular lumen, where it binds to an anion, usually phosphate, and is excreted into the urine.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.
Description
Day 1: The Renal System: Structure and Function & Overview of Renal Pathophysiology ppt Day 2: Glomerular & Tubular Disorders ppt Day 3: The Renal System: Osmoregulation Acid-base Homeostasis ppt