Podcast
Questions and Answers
What is a functional group?
What is a functional group?
A functional group is a certain pattern of atoms in an organic molecule.
Name the first 6 alcohols.
Name the first 6 alcohols.
Methanol, Ethanol, Propanol, Butanol, Pentanol, Hexanol.
Name the first 6 carboxylic acids.
Name the first 6 carboxylic acids.
Formic acid, Acetic acid, Propanoic acid, Butanoic acid, Pentanoic acid, Hexanoic acid.
What is the structural formula for Pentene?
What is the structural formula for Pentene?
What is the molecular formula for Ethyne?
What is the molecular formula for Ethyne?
What is the molecular formula for Butane?
What is the molecular formula for Butane?
How many covalent bonds can carbon form?
How many covalent bonds can carbon form?
How many covalent bonds can hydrogen form?
How many covalent bonds can hydrogen form?
How many covalent bonds will oxygen form?
How many covalent bonds will oxygen form?
How many covalent bonds will nitrogen form?
How many covalent bonds will nitrogen form?
Carboxylic acids are strong acids.
Carboxylic acids are strong acids.
What is the functional group of alcohols?
What is the functional group of alcohols?
Which acid is found in vinegar?
Which acid is found in vinegar?
Esters are often found in which of the following?
Esters are often found in which of the following?
Flashcards are hidden until you start studying
Study Notes
Alcohols
- Alcohols contain a hydroxyl functional group (-OH).
- They are named based on the number of carbon atoms with the suffix “-anol”.
- For example, pentanol contains five carbon atoms.
- Ethanol, the alcohol found in alcoholic beverages, can also serve as a fuel source.
- Fermentation, a process involving yeast, converts sugar into ethanol.
Carboxylic Acids
- Carboxylic acids have a carboxyl functional group (-COOH).
- The name of the carboxylic acid is based on the number of carbon atoms followed by the suffix “-anoic acid”.
- For example, pentanoic acid comprises five carbon atoms.
- These acids tend to possess a foul odour.
- Propanoic acid contributes to the smell of sweat, while butanoic acid is associated with spoiled butter.
- Ethanoic acid, commonly found in vinegar, is a weak acid that releases hydrogen ions when dissolved in water.
Esters
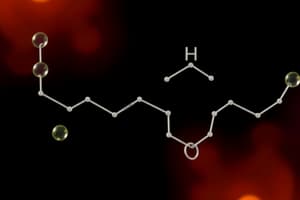
- Esters feature a C-CO2-C functional group. They are often found in fruits, contributing to their fragrance and flavour.
- Esters can be synthesized by reacting a carboxylic acid with an alcohol.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.