Podcast
Questions and Answers
What is the rise per base pair in B-DNA?
What is the rise per base pair in B-DNA?
- 5.6A
- 2.5A
- 4.5A
- 3.4A (correct)
What is the characteristic of Z-form DNA?
What is the characteristic of Z-form DNA?
- Single-stranded DNA
- Double-stranded RNA
- Right-handed helix
- Left-handed helix (correct)
What is the function of histone amino terminal tails?
What is the function of histone amino terminal tails?
- To transcribe genes
- To compact chromatin (correct)
- To replicate DNA
- To unwind DNA
What is the function of the protein H1?
What is the function of the protein H1?
What is the next stage in the organisation of DNA after the 10nm fibre?
What is the next stage in the organisation of DNA after the 10nm fibre?
What determines the size of DNA loops?
What determines the size of DNA loops?
What is the role of SMC proteins in DNA loop formation?
What is the role of SMC proteins in DNA loop formation?
What is the structure formed by chromosomal DNA bound to proteins like histones and other DNA-packaging factors?
What is the structure formed by chromosomal DNA bound to proteins like histones and other DNA-packaging factors?
What is the main objective of the MedST/VetST Part IA Molecules in Medical Sciences course?
What is the main objective of the MedST/VetST Part IA Molecules in Medical Sciences course?
Who is the lecturer for the MedST/VetST Part IA Molecules in Medical Sciences course?
Who is the lecturer for the MedST/VetST Part IA Molecules in Medical Sciences course?
What is the title of the course book for the MedST/VetST Part IA Molecules in Medical Sciences course?
What is the title of the course book for the MedST/VetST Part IA Molecules in Medical Sciences course?
What is the focus of Dr. Helen Mott's research group?
What is the focus of Dr. Helen Mott's research group?
What is the definition of a living cell, according to the lecture?
What is the definition of a living cell, according to the lecture?
What is the purpose of the NCBI bookshelf mentioned in the lecture?
What is the purpose of the NCBI bookshelf mentioned in the lecture?
How many lectures are in the MedST/VetST Part IA Molecules in Medical Sciences course?
How many lectures are in the MedST/VetST Part IA Molecules in Medical Sciences course?
What is the focus of the fifth objective of the MedST/VetST Part IA Molecules in Medical Sciences course?
What is the focus of the fifth objective of the MedST/VetST Part IA Molecules in Medical Sciences course?
What is the primary function of carbohydrates in biological systems?
What is the primary function of carbohydrates in biological systems?
What are the two common sugars found in biological systems?
What are the two common sugars found in biological systems?
What is the term for the process by which sugars are linked together?
What is the term for the process by which sugars are linked together?
What is the characteristic of the energy storage carbohydrate, glycogen?
What is the characteristic of the energy storage carbohydrate, glycogen?
What is the function of nucleic acids in biological systems?
What is the function of nucleic acids in biological systems?
What are the components of a nucleotide?
What are the components of a nucleotide?
What is the term for the complex carbohydrates linked to proteins on the cell surface?
What is the term for the complex carbohydrates linked to proteins on the cell surface?
What is the result of impaired glucose regulation in the body?
What is the result of impaired glucose regulation in the body?
What is the term for the process by which sugars adopt a cyclic configuration?
What is the term for the process by which sugars adopt a cyclic configuration?
What is the function of carbohydrates in plant cell walls?
What is the function of carbohydrates in plant cell walls?
What are the four different bases found in DNA?
What are the four different bases found in DNA?
What type of bonds hold nucleotides together in a nucleic acid?
What type of bonds hold nucleotides together in a nucleic acid?
What is the term for the structure of DNA where two complementary chains bind together?
What is the term for the structure of DNA where two complementary chains bind together?
What is the function of the ribosome?
What is the function of the ribosome?
What is the term for the description of the order of amino acid residues within a protein chain?
What is the term for the description of the order of amino acid residues within a protein chain?
What is the term for the overall three-dimensional fold of a protein molecule?
What is the term for the overall three-dimensional fold of a protein molecule?
What is the term for the description of the arrangement of multiple amino acid chains in a protein?
What is the term for the description of the arrangement of multiple amino acid chains in a protein?
What is the characteristic of the hydrophobic side chains in amino acids?
What is the characteristic of the hydrophobic side chains in amino acids?
How many amino acids are commonly found in proteins?
How many amino acids are commonly found in proteins?
What is the chiral form of amino acids found in natural proteins?
What is the chiral form of amino acids found in natural proteins?
What type of bonds are strong and form between oppositely charged chemical groups in an aqueous environment?
What type of bonds are strong and form between oppositely charged chemical groups in an aqueous environment?
What is the reason for the increase in strength of electrostatic interactions in the hydrophobic core of a protein?
What is the reason for the increase in strength of electrostatic interactions in the hydrophobic core of a protein?
What type of interactions occur between all atoms and are individually weak but collectively contribute to considerable energy to the structure of a protein?
What type of interactions occur between all atoms and are individually weak but collectively contribute to considerable energy to the structure of a protein?
Where do disulphide bonds typically form in proteins?
Where do disulphide bonds typically form in proteins?
What is the driving force for protein folding?
What is the driving force for protein folding?
What type of proteins are usually structural molecules such as keratin or collagen?
What type of proteins are usually structural molecules such as keratin or collagen?
What is the characteristic of the 'hydrophobic effect' in globular proteins?
What is the characteristic of the 'hydrophobic effect' in globular proteins?
What is the term for the smaller pieces of tertiary structures of proteins?
What is the term for the smaller pieces of tertiary structures of proteins?
What is an example of a motif in proteins?
What is an example of a motif in proteins?
What is the purpose of ribbon diagrams in representing protein structures?
What is the purpose of ribbon diagrams in representing protein structures?
What is unique about cysteine side chains in proteins?
What is unique about cysteine side chains in proteins?
What is the purpose of the peptide bond in proteins?
What is the purpose of the peptide bond in proteins?
What is responsible for the planar structure of the peptide bond?
What is responsible for the planar structure of the peptide bond?
What determines the conformation of the backbone of a protein?
What determines the conformation of the backbone of a protein?
What is the Ramachandran plot used for?
What is the Ramachandran plot used for?
What determines the overall 3D structure and function of a protein?
What determines the overall 3D structure and function of a protein?
What is the result of adding urea and mercaptoethanol to a protein?
What is the result of adding urea and mercaptoethanol to a protein?
What is necessary for a protein to regain its native 3D structure and function?
What is necessary for a protein to regain its native 3D structure and function?
What is the significance of the hydrophilic side chains in proteins?
What is the significance of the hydrophilic side chains in proteins?
What is the role of the peptide bond in the formation of the protein structure?
What is the role of the peptide bond in the formation of the protein structure?
What is the primary level of protein folding?
What is the primary level of protein folding?
What type of protein structure is formed by 8 β-strands?
What type of protein structure is formed by 8 β-strands?
Which type of interaction is responsible for stabilising the tertiary structure of a protein?
Which type of interaction is responsible for stabilising the tertiary structure of a protein?
What is the directionality of hydrogen bonds in protein structure?
What is the directionality of hydrogen bonds in protein structure?
What is the result of dissolving hydrophobic groups in water?
What is the result of dissolving hydrophobic groups in water?
What is the role of the hydrophobic effect in protein structure?
What is the role of the hydrophobic effect in protein structure?
What is the main component of a protein's 'hydrophobic core'?
What is the main component of a protein's 'hydrophobic core'?
What is the primary function of hydrogen bonds in protein structure?
What is the primary function of hydrogen bonds in protein structure?
What is the characteristic of a protein domain?
What is the characteristic of a protein domain?
What is the main function of an antibody?
What is the main function of an antibody?
What is the term for the structure formed by the way different subunits of a protein fit together?
What is the term for the structure formed by the way different subunits of a protein fit together?
What is the component of an antibody that binds to a specific surface of an antigen?
What is the component of an antibody that binds to a specific surface of an antigen?
How many immunoglobulin domains are found in each antibody heavy chain?
How many immunoglobulin domains are found in each antibody heavy chain?
What is the function of disulphide bonds in immunoglobulin domains?
What is the function of disulphide bonds in immunoglobulin domains?
What is the term for the structure of a protein that consists of a four-stranded antiparallel beta-sheet and a three-stranded anti-parallel beta-sheet?
What is the term for the structure of a protein that consists of a four-stranded antiparallel beta-sheet and a three-stranded anti-parallel beta-sheet?
What is the characteristic of a multidomain protein?
What is the characteristic of a multidomain protein?
What is the function of B lymphocytes in the immune system?
What is the function of B lymphocytes in the immune system?
What is the term for the process by which a lymphocyte that produces a specific antibody is triggered to replicate, increasing the amount of the specific antibody in the blood?
What is the term for the process by which a lymphocyte that produces a specific antibody is triggered to replicate, increasing the amount of the specific antibody in the blood?
What is the region of the antibody responsible for interactions with the immune system?
What is the region of the antibody responsible for interactions with the immune system?
What is the result of proteolytic cleavage of the hinge region in an antibody?
What is the result of proteolytic cleavage of the hinge region in an antibody?
What is the function of the Fc portion of an antibody?
What is the function of the Fc portion of an antibody?
What are the hypervariable regions of the antibody known as?
What are the hypervariable regions of the antibody known as?
What is the purpose of the CDRs in an antibody?
What is the purpose of the CDRs in an antibody?
What is the shape of the recognition site in antibodies that bind to large macromolecules?
What is the shape of the recognition site in antibodies that bind to large macromolecules?
What type of interactions hold the complex between the antibody and antigen together?
What type of interactions hold the complex between the antibody and antigen together?
What is the function of the Fab fragments of an antibody?
What is the function of the Fab fragments of an antibody?
What is the structure of the light chain in an antibody?
What is the structure of the light chain in an antibody?
What is the purpose of the framework in the variable domains of an antibody?
What is the purpose of the framework in the variable domains of an antibody?
What is the primary function of cofactors in proteins?
What is the primary function of cofactors in proteins?
What is the key difference between a cofactor and a prosthetic group?
What is the key difference between a cofactor and a prosthetic group?
What is the purpose of NADH in the reduction of pyruvate to lactate?
What is the purpose of NADH in the reduction of pyruvate to lactate?
What is the characteristic of the bonds that hold the protein structure together?
What is the characteristic of the bonds that hold the protein structure together?
What is the result of the flexibility of protein molecules?
What is the result of the flexibility of protein molecules?
What is the function of the haem group in myoglobin and haemoglobin?
What is the function of the haem group in myoglobin and haemoglobin?
What is the purpose of cosubstrates in protein function?
What is the purpose of cosubstrates in protein function?
What is the characteristic of the nicotinamide ring in NADH?
What is the characteristic of the nicotinamide ring in NADH?
What is the primary function of myoglobin in vertebrate muscle?
What is the primary function of myoglobin in vertebrate muscle?
What is the name of the prosthetic group found in myoglobin and haemoglobin?
What is the name of the prosthetic group found in myoglobin and haemoglobin?
What is the characteristic of the oxygen binding curve of myoglobin?
What is the characteristic of the oxygen binding curve of myoglobin?
What is the term for the phenomenon where binding of ligand to one site influences the affinity of binding to other sites on the same molecule?
What is the term for the phenomenon where binding of ligand to one site influences the affinity of binding to other sites on the same molecule?
What is the result of oxygen binding to haemoglobin?
What is the result of oxygen binding to haemoglobin?
What is the significance of the sigmoidal binding properties of haemoglobin?
What is the significance of the sigmoidal binding properties of haemoglobin?
What is the term for the movement of the Fe(II) ion into the centre of the porphyrin ring during oxygen binding to haemoglobin?
What is the term for the movement of the Fe(II) ion into the centre of the porphyrin ring during oxygen binding to haemoglobin?
What is the effect of oxygen binding on the interface between subunits in haemoglobin?
What is the effect of oxygen binding on the interface between subunits in haemoglobin?
What is the quaternary structure of haemoglobin?
What is the quaternary structure of haemoglobin?
In low oxygen conditions, what property of haemoglobin allows it to release oxygen to the tissue?
In low oxygen conditions, what property of haemoglobin allows it to release oxygen to the tissue?
What is the role of the histidine residue in haemoglobin?
What is the role of the histidine residue in haemoglobin?
What is the main difference between soluble proteins and membrane proteins?
What is the main difference between soluble proteins and membrane proteins?
What is the function of the potassium channel?
What is the function of the potassium channel?
What is the structure of the lipid molecules in a biological membrane?
What is the structure of the lipid molecules in a biological membrane?
What is the purpose of the selectivity filter in the potassium channel?
What is the purpose of the selectivity filter in the potassium channel?
What is the structure of the potassium channel?
What is the structure of the potassium channel?
What is the purpose of the a-helices in membrane proteins?
What is the purpose of the a-helices in membrane proteins?
What is the function of membrane proteins in biological membranes?
What is the function of membrane proteins in biological membranes?
What is the main difference between the structure of a soluble protein and a membrane protein?
What is the main difference between the structure of a soluble protein and a membrane protein?
What is the term for the hydrophilic path through the centre of the potassium channel?
What is the term for the hydrophilic path through the centre of the potassium channel?
What is the function of the oxygen atoms in the selectivity filter of a potassium channel?
What is the function of the oxygen atoms in the selectivity filter of a potassium channel?
What is the purpose of X-ray crystallography in determining protein structure?
What is the purpose of X-ray crystallography in determining protein structure?
Why does the potassium channel allow a thousand potassium ions through for every sodium ion?
Why does the potassium channel allow a thousand potassium ions through for every sodium ion?
What is the main difference between X-ray crystallography and Nuclear Magnetic Resonance (NMR) spectroscopy in determining protein structure?
What is the main difference between X-ray crystallography and Nuclear Magnetic Resonance (NMR) spectroscopy in determining protein structure?
What is the purpose of chromatography in protein purification?
What is the purpose of chromatography in protein purification?
What is the function of the selectivity filter in a potassium channel?
What is the function of the selectivity filter in a potassium channel?
What is the purpose of gel filtration in protein purification?
What is the purpose of gel filtration in protein purification?
What happens to larger proteins during gel filtration chromatography?
What happens to larger proteins during gel filtration chromatography?
What is the purpose of the Bradford assay?
What is the purpose of the Bradford assay?
Why is cryoelectron microscopy used to solve structures of large proteins and assemblies?
Why is cryoelectron microscopy used to solve structures of large proteins and assemblies?
What is the purpose of ion exchange chromatography in protein purification?
What is the purpose of ion exchange chromatography in protein purification?
What is the function of zinc fingers in DNA binding proteins?
What is the function of zinc fingers in DNA binding proteins?
What is the first stage in protein structure determination?
What is the first stage in protein structure determination?
What is the result of misfolded PrP proteins in prion diseases?
What is the result of misfolded PrP proteins in prion diseases?
What is the purpose of SDS PAGE?
What is the purpose of SDS PAGE?
What is the term for the process by which proteins misfold and form insoluble, fibrous aggregates?
What is the term for the process by which proteins misfold and form insoluble, fibrous aggregates?
What is the function of bioinformatics in the analysis of primary structure?
What is the function of bioinformatics in the analysis of primary structure?
What is the driving force behind the formation of protein structure?
What is the driving force behind the formation of protein structure?
What is the characteristic of a zinc finger motif?
What is the characteristic of a zinc finger motif?
What is the consequence of misfolded proteins in Alzheimer's disease?
What is the consequence of misfolded proteins in Alzheimer's disease?
What is the purpose of SDS in the polyacrylamide gel electrophoresis process?
What is the purpose of SDS in the polyacrylamide gel electrophoresis process?
What is the purpose of the antibody linked to an enzyme in an ELISA assay?
What is the purpose of the antibody linked to an enzyme in an ELISA assay?
What is the purpose of the molecular weight markers in an SDS-PAGE gel?
What is the purpose of the molecular weight markers in an SDS-PAGE gel?
What is the characteristic of enzyme-catalyzed reactions?
What is the characteristic of enzyme-catalyzed reactions?
What is the primary reason enzymes are suited as drug targets?
What is the primary reason enzymes are suited as drug targets?
What do enzymes overcome in a reaction?
What do enzymes overcome in a reaction?
What is the purpose of the antibody in an ELISA assay?
What is the purpose of the antibody in an ELISA assay?
What is the function of the electric current in SDS-PAGE?
What is the function of the electric current in SDS-PAGE?
What is the balance of enthalpy and entropy changes determined by?
What is the balance of enthalpy and entropy changes determined by?
What is the point at which the forwards rate of a reaction is equal to the backwards rate?
What is the point at which the forwards rate of a reaction is equal to the backwards rate?
What is the purpose of Coomassie Blue stain in SDS-PAGE?
What is the purpose of Coomassie Blue stain in SDS-PAGE?
What is related to the final equilibrium position of a chemical reaction?
What is related to the final equilibrium position of a chemical reaction?
What is the characteristic of enzymes?
What is the characteristic of enzymes?
What is the term for the free energy of a reaction under standard conditions?
What is the term for the free energy of a reaction under standard conditions?
What is the purpose of ELISA assays in hospital diagnosis?
What is the purpose of ELISA assays in hospital diagnosis?
What is the purpose of enzymes in controlling glucose metabolism?
What is the purpose of enzymes in controlling glucose metabolism?
What is the characteristic of the enzyme-linked immunosorbent assay (ELISA)?
What is the characteristic of the enzyme-linked immunosorbent assay (ELISA)?
What is the result of impaired glucose regulation in the body?
What is the result of impaired glucose regulation in the body?
What is an example of a successful drug that inhibits the function of an enzyme from a pathogenic organism?
What is an example of a successful drug that inhibits the function of an enzyme from a pathogenic organism?
What is the term for the ratio of the concentrations of reactants and products at equilibrium?
What is the term for the ratio of the concentrations of reactants and products at equilibrium?
What is the unit of concentration at which the free energy is defined?
What is the unit of concentration at which the free energy is defined?
What is the purpose of coupling reactions in biological systems?
What is the purpose of coupling reactions in biological systems?
What is the role of ATP in reaction coupling?
What is the role of ATP in reaction coupling?
What is the transition state in a reaction?
What is the transition state in a reaction?
How do enzymes reduce the energy barrier of a reaction?
How do enzymes reduce the energy barrier of a reaction?
What is the location where the reaction takes place in an enzyme?
What is the location where the reaction takes place in an enzyme?
What is the equation that relates the free energy change to the equilibrium constant?
What is the equation that relates the free energy change to the equilibrium constant?
What is the purpose of ATP hydrolysis in reaction coupling?
What is the purpose of ATP hydrolysis in reaction coupling?
What is the kinetic barrier that prevents a reaction from proceeding?
What is the kinetic barrier that prevents a reaction from proceeding?
What is the effect of an enzyme on the energy of the transition state?
What is the effect of an enzyme on the energy of the transition state?
What is the primary function of the active site of an enzyme?
What is the primary function of the active site of an enzyme?
What is responsible for the distortion of the substrate molecule into the transition state?
What is responsible for the distortion of the substrate molecule into the transition state?
What is the purpose of the active site having a shape complementary to the transition state?
What is the purpose of the active site having a shape complementary to the transition state?
What is the result of the interactions between the active site and the transition state?
What is the result of the interactions between the active site and the transition state?
What is the role of electrostatic groups and hydrogen bond donors and acceptors in the active site?
What is the role of electrostatic groups and hydrogen bond donors and acceptors in the active site?
What is the purpose of the enzyme bringing substrate molecules together in close proximity?
What is the purpose of the enzyme bringing substrate molecules together in close proximity?
What determines the shape of the active site?
What determines the shape of the active site?
What is the result of the enzyme's precise arrangement of the active site?
What is the result of the enzyme's precise arrangement of the active site?
What is the role of Asp1 in the reactive cycle?
What is the role of Asp1 in the reactive cycle?
What is the purpose of targeting HIV protease in the design of inhibitors?
What is the purpose of targeting HIV protease in the design of inhibitors?
What is the characteristic of HIV protease inhibitors?
What is the characteristic of HIV protease inhibitors?
What is the function of the protease in the HIV life cycle?
What is the function of the protease in the HIV life cycle?
What is the mechanism of action of HIV protease inhibitors?
What is the mechanism of action of HIV protease inhibitors?
What is the importance of the knowledge of the specificity of the binding pocket in the design of HIV protease inhibitors?
What is the importance of the knowledge of the specificity of the binding pocket in the design of HIV protease inhibitors?
What is the classification of the enzyme alcohol dehydrogenase?
What is the classification of the enzyme alcohol dehydrogenase?
What is the function of enzymes in biological systems?
What is the function of enzymes in biological systems?
What is the characteristic of the Asp2 in the reactive cycle?
What is the characteristic of the Asp2 in the reactive cycle?
What is the outcome of the use of HIV protease inhibitors in treatment?
What is the outcome of the use of HIV protease inhibitors in treatment?
What is the role of the specificity pocket in serine proteases?
What is the role of the specificity pocket in serine proteases?
What is the function of the oxyanion hole in serine proteases?
What is the function of the oxyanion hole in serine proteases?
What is the role of the charge-relay mechanism in serine proteases?
What is the role of the charge-relay mechanism in serine proteases?
What is the function of the catalytic serine residue in serine proteases?
What is the function of the catalytic serine residue in serine proteases?
What is the role of His57 in serine proteases?
What is the role of His57 in serine proteases?
What is the difference between serine proteases and Asp proteases?
What is the difference between serine proteases and Asp proteases?
What is the result of the first stage of the reaction mechanism in serine proteases?
What is the result of the first stage of the reaction mechanism in serine proteases?
What is the role of the acyl-enzyme intermediate in serine proteases?
What is the role of the acyl-enzyme intermediate in serine proteases?
What is the function of the water molecule in the second stage of the reaction mechanism in serine proteases?
What is the function of the water molecule in the second stage of the reaction mechanism in serine proteases?
What is the overall effect of the serine protease mechanism on the reaction rate?
What is the overall effect of the serine protease mechanism on the reaction rate?
What is the primary function of enzymes in a biological system?
What is the primary function of enzymes in a biological system?
What is the main effect of the zinc ion in the active site of carbonic anhydrase?
What is the main effect of the zinc ion in the active site of carbonic anhydrase?
What is the role of the histidine residue (His 64) in carbonic anhydrase?
What is the role of the histidine residue (His 64) in carbonic anhydrase?
What is the term for the model of enzyme function that suggests the active site and substrate undergo deformation upon binding?
What is the term for the model of enzyme function that suggests the active site and substrate undergo deformation upon binding?
What is the function of the serine proteases in the cell?
What is the function of the serine proteases in the cell?
What is the purpose of the active site in an enzyme?
What is the purpose of the active site in an enzyme?
What is the term for the process of stabilising the transition state in an enzyme-catalysed reaction?
What is the term for the process of stabilising the transition state in an enzyme-catalysed reaction?
What is the role of the flexible loop in hexokinase?
What is the role of the flexible loop in hexokinase?
What is the purpose of the binding pocket in the active site of carbonic anhydrase?
What is the purpose of the binding pocket in the active site of carbonic anhydrase?
What is the characteristic of the active site in the lock-and-key model?
What is the characteristic of the active site in the lock-and-key model?
What is the significance of measuring initial rates of chemical reactions?
What is the significance of measuring initial rates of chemical reactions?
What is the relationship between v0 and [S] according to the Michaelis-Menten equation?
What is the relationship between v0 and [S] according to the Michaelis-Menten equation?
What is the significance of vMAX in the Michaelis-Menten equation?
What is the significance of vMAX in the Michaelis-Menten equation?
What is the significance of KM in the Michaelis-Menten equation?
What is the significance of KM in the Michaelis-Menten equation?
What is the purpose of the Lineweaver-Burk plot?
What is the purpose of the Lineweaver-Burk plot?
What is the effect of activators on enzyme activity?
What is the effect of activators on enzyme activity?
What is the effect of inhibitors on enzyme activity?
What is the effect of inhibitors on enzyme activity?
What is the significance of k2 in the derivation of the Michaelis-Menten equation?
What is the significance of k2 in the derivation of the Michaelis-Menten equation?
What is the assumption in the derivation of the Michaelis-Menten equation?
What is the assumption in the derivation of the Michaelis-Menten equation?
What is the significance of the ES complex in the Michaelis-Menten equation?
What is the significance of the ES complex in the Michaelis-Menten equation?
What type of chemical reaction is catalyzed by hydrolases?
What type of chemical reaction is catalyzed by hydrolases?
What is the role of protein kinase in enzyme classification?
What is the role of protein kinase in enzyme classification?
What is the primary function of enzymes in a cell?
What is the primary function of enzymes in a cell?
What is the significance of enzyme kinetics in understanding cellular processes?
What is the significance of enzyme kinetics in understanding cellular processes?
What is the simplest type of enzyme kinetics described by?
What is the simplest type of enzyme kinetics described by?
What is the characteristic of a ligase enzyme?
What is the characteristic of a ligase enzyme?
What is the role of aldolase in glycolysis?
What is the role of aldolase in glycolysis?
What is the significance of studying enzyme kinetics in the context of disease?
What is the significance of studying enzyme kinetics in the context of disease?
What is the difference between the kinetics of hexokinase and glucokinase?
What is the difference between the kinetics of hexokinase and glucokinase?
What is the assumption made in the Michaelis-Menten equation?
What is the assumption made in the Michaelis-Menten equation?
What is the purpose of a competitive inhibitor?
What is the purpose of a competitive inhibitor?
What is the effect of a competitive inhibitor on the vMAX of a reaction?
What is the effect of a competitive inhibitor on the vMAX of a reaction?
What is a characteristic of transition state analogues?
What is a characteristic of transition state analogues?
What is the purpose of a Lineweaver-Burk plot?
What is the purpose of a Lineweaver-Burk plot?
How can you determine if an inhibitor is acting competitively?
How can you determine if an inhibitor is acting competitively?
What is the effect of a competitive inhibitor on the KM of a reaction?
What is the effect of a competitive inhibitor on the KM of a reaction?
What is the characteristic of noncompetitive inhibition?
What is the characteristic of noncompetitive inhibition?
What is the purpose of an allosteric inhibitor?
What is the purpose of an allosteric inhibitor?
What is the term for the analysis of the kinetics and determination of KM and VMAX of a reaction?
What is the term for the analysis of the kinetics and determination of KM and VMAX of a reaction?
What is the characteristic of HIV protease inhibitors?
What is the characteristic of HIV protease inhibitors?
What type of inhibition does saquinavir exhibit?
What type of inhibition does saquinavir exhibit?
What is the effect of irreversible inhibition on an enzyme?
What is the effect of irreversible inhibition on an enzyme?
What is the function of acetylcholine esterase in the neuromuscular junction?
What is the function of acetylcholine esterase in the neuromuscular junction?
What is the mechanism of action of suicide inhibitors?
What is the mechanism of action of suicide inhibitors?
What is the result of phosphorylation of Tyr15 in the active site of cdk2?
What is the result of phosphorylation of Tyr15 in the active site of cdk2?
What is the purpose of reversible covalent modification in enzyme control?
What is the purpose of reversible covalent modification in enzyme control?
What is the characteristic of zymogens?
What is the characteristic of zymogens?
What is the effect of penicillin on bacterial cell wall synthesis?
What is the effect of penicillin on bacterial cell wall synthesis?
What is the purpose of phosphatases in enzyme regulation?
What is the purpose of phosphatases in enzyme regulation?
What is the result of the reaction between sarin gas and acetylcholine esterase?
What is the result of the reaction between sarin gas and acetylcholine esterase?
Flashcards are hidden until you start studying
Study Notes
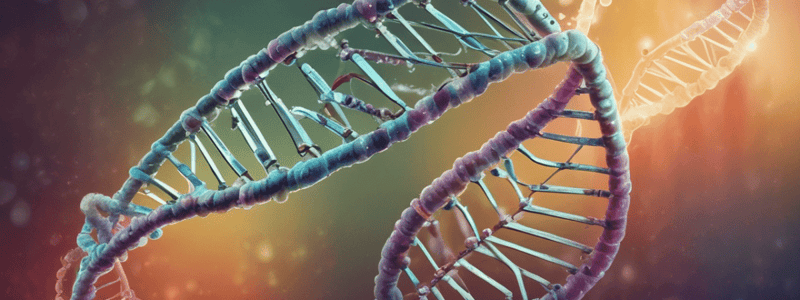
DNA Structure
- DNA strands are joined by phosphodiester bonds, with negatively charged phosphates facing outwards
- One turn of the helix occurs every 10.5 bases in B-DNA
- The rise per base pair is 3.4Å, and the width of the helix is 20Å
- The rise per helical turn is 10.5 x 3.4Å = 35.7Å
Forms of DNA
- B-form is the most common, while dsRNA adopts the A-form, which is wider than B-form with a more compressed major groove, wider minor groove, and tilted bases
- GC-rich DNA can adopt the A-form
- Z-form is a left-handed DNA, where the phosphate backbone follows a zigzag trajectory
Chromatin Structure
- Chromatin is formed by chromosomal DNA bound to proteins like histones and other DNA-packaging factors
- The basic unit of chromatin is the nucleosome
- The nucleosome core particle consists of a sequence of 146nt wrapped around a barrel-shaped octamer of histone in 1.7 turns of a left-handed superhelix
Histones and Nucleosome
- Histones are positively charged proteins
- The histone octamer of the NCP particle consists of 2 H2A-H2B dimers flanking a tetramer of 2 H3 and H4
- The amino terminal tails of histones project and can be modified, leading to compaction of chromatin and control of gene transcription
- NCP can interact with H1, which binds nucleosomal DNA at entry/exit points in NCP and compacts more
Chromatin Fibers
- The 10nm fibre is the most abundant chromatin form, with NCP separated by linker DNA, and a repeat length of nucleosome = 200nt
- The 10nm fibre can transition to the 30nm fibre (regular/compact form), which is most folds randomly
DNA Loop Formation
- DNA loops are the next stage in the organisation of DNA, anchored to protein-chromosome scaffold, and segregated from the genome
- DNA loops bring enhancers and promoters together, and are important in gene regulation during development and differentiation
- Structural Maintenance of Chromosome (SMC) proteins, such as cohesin and condensin, have a split ATPase domain, and play a role in DNA loop formation
- Cohesin and condensin heads are linked by non-SMC subunit, giving a topological ring, trapping DNA, and forming a loop
- The size of DNA loops is determined by CTCF binding sites, which recruits cohesin
Biological Macromolecules
- Biological macromolecules include carbohydrates, nucleic acids, and proteins
- These molecules interact and react in a dynamic network within a living cell
Carbohydrates
- Provide and store energy
- Offer structural support in plant and bacterial cell walls
- Play a role in cellular signalling, cell motility, and cell-cell adhesion
- Medically important, e.g., glucose is the main source of energy in the body
- Diabetes occurs when the body's ability to regulate glucose is impaired
- Glycogen storage diseases occur when enzymes needed to convert glucose into glycogen are lacking
Structure of Carbohydrates
- Formed by linking together sugars (monosaccharides) into chains
- Chains can be linear or branched
- Sugars can adopt a cyclic configuration
- Glucose and fructose are two common sugars
- Two forms of glucose: α- and β-glucose
- Condensation reactions form disaccharides (e.g., sucrose) and polysaccharides (e.g., cellulose)
Nucleic Acids
- Essential for cellular information storage
- DNA is the primary information store in most cells
- RNA plays a central role in gene expression and is the information store for many viruses
- Nucleic acids are formed by the polymerization of nucleotides
- Nucleotides consist of a sugar, phosphate, and base
- Four bases found in DNA: adenine, thymine, guanine, and cytosine
- RNA uses uracil instead of thymine
- Nucleotides are linked through phosphodiester bonds
Protein Structure
- Primary structure: the order of amino acid residues within a chain
- Secondary structure: localized, regular arrangements of the polypeptide backbone
- Tertiary structure: the overall three-dimensional fold of the protein
- Quaternary structure: the arrangement of multiple amino acid chains
Amino Acids
- Chiral molecules consisting of a central carbon atom, amino group, carboxyl group, hydrogen, and side chain (R group)
- 20 amino acids commonly found in proteins
- Side chains determine protein function and folding
- Hydrophobic side chains cluster together, away from water
- Hydrophilic side chains form strong hydrogen bonds with water
Peptide Bond
- Formed through condensation reactions between amino acids
- Planar structure with partial double bond characteristics
- Restricts rotational movement of the polypeptide chain
Folding of the Polypeptide Chain
- Occurs by rotation of the torsion angles for each residue
- Ramachandran plot shows the combinations of angles found in protein structures
- Dark grey areas are sterically allowed, while lighter grey areas are sterically strained but still allowed
Secondary Structure
- α-Helix: a single, right-handed helix formed through hydrogen bonding between the N-H and C=O groups of the peptide bond
- β-Sheet: formed through hydrogen bonding between the N-H and C=O groups of the peptide bond
- Much of a protein chain forms secondary structure to maximize hydrogen bonding interactions### Protein Structure
- Proteins have a tightly wound, ordered structure that provides strength
- Globular proteins have compact structures with hydrophobic side chains clustering in the centre and hydrophilic side chains on the surface
- The hydrophobic effect is a major driving force for folding of globular proteins
Representation of Protein Structure
- Protein structures can be represented in many ways, including ribbon diagrams, topology diagrams, and 3D models
- Ribbon diagrams show α-helices as cylinders and β-sheets as flat arrows
- Topology diagrams help to understand the connectivity and path of the protein chain through the structure
Motifs and Domains
- Motifs, or super-secondary structures, are groupings of secondary structural elements that are not structurally independent units
- Domains are larger than motifs and form compact units with a globular core
- Multidomain proteins contain multiple domains formed from a single polypeptide chain
Quaternary Structure
- Many proteins are made of multiple polypeptide chains, with each chain forming one subunit of the assembly
- The way in which the different subunits fit together is described by the quaternary structure
Antibodies
- Antibodies, or immunoglobulins, are proteins used by the immune system for molecular recognition
- Antibodies are tetrameric molecules consisting of two identical light chains and two identical heavy chains
- Each antibody has a unique variable domain that recognizes a specific antigen
- The constant domains of the heavy and light chains are responsible for interactions with the immune system
Antibody Structure
- Antibody heavy chains have three relatively conserved immunoglobulin domains and an N-terminal variable domain
- Antibody light chains have one constant domain and one N-terminal variable domain
- Disulphide bonds stabilize individual domains and form covalent linkages to join the four chains in a stable complex
Antibody Function
- Antibodies bind to specific antigens through the variable domains
- The antibody surface forms a recognition site that is complementary in shape to the epitope
- Antibodies can recognize a wide range of antigens, from small molecules to large proteins
Cofactors and Prosthetic Groups
- Cofactors are molecules that provide chemical reactivity not available from amino acids
- Cofactors can be loosely bound (cosubstrate) or tightly bound (prosthetic group) to the protein
- Examples of cofactors:
- NADH and NADPH: used as cosubstrates to perform reduction processes
- Haem group: a prosthetic group in myoglobin and haemoglobin, involved in oxygen binding
- Prosthetic groups are often metal ions or organic molecules that are covalently attached to the protein
Protein Flexibility and Conformational Changes
- Proteins are highly dynamic structures in solution, with rapid fluctuations
- Flexibility comes from the nature of the bonds holding the protein structure together
- Weak and non-directional bonds (e.g. hydrophobic effect, Van der Waals forces, electrostatic interactions) allow for small movements without affecting the strength of the bonding interactions
- Conformational changes are important for protein function, and can be stabilised by binding of small molecules or phosphorylation
Myoglobin and Haemoglobin
- Myoglobin: a small monomeric protein found in vertebrate muscle, involved in oxygen storage and transport
- Haemoglobin: a tetrameric protein involved in oxygen transport in the blood
- Both proteins have a similar tertiary structure, with a buried haem group that binds oxygen
- Haemoglobin shows cooperativity, with the binding of oxygen to one subunit affecting the oxygen affinity of the other subunits
Membrane Proteins
- Membranes are formed of lipid molecules, with a hydrophilic head group and hydrophobic fatty acid tails
- Membrane proteins are adapted to the membrane environment, with a hydrophobic external surface and a hydrophilic internal surface
- Membrane-spanning parts of proteins are often α-helical or β-barrel structures
- Ion channels, such as the potassium channel, provide a hydrophilic path through the membrane for ions to pass through
Determination of Protein Structure
- Techniques used to determine protein structure:
- X-ray crystallography
- Nuclear magnetic resonance (NMR) spectroscopy
- Cryoelectron microscopy
- X-ray crystallography involves:
- Crystallising the protein
- Diffracting X-rays through the crystal
- Using Fourier transforms to calculate the electron density of the protein
- Building a model of the protein through the electron density
Protein Purification
- Steps involved in protein purification:
- Fractionation techniques (e.g. chromatography)
- Affinity chromatography: uses a molecule that binds specifically to the protein of interest
- Ion exchange chromatography: separates proteins by charge
- Gel filtration: separates proteins by size
- Bioinformatics: the analysis of primary structure (amino acid sequence) to predict protein structure and function
Misfolded Proteins
- Misfolded proteins can lead to diseases, such as Alzheimer's and prion diseases
- In Alzheimer's disease, a 40-42 residue fragment from a membrane protein forms amyloid fibrils
- In prion diseases, the prion protein (PrP) misfolds to form toxic fibrils, leading to neural degeneration
Protein Analysis
- Techniques used to analyse proteins:
- Bradford assay: measures the total amount of protein in a sample
- SDS-PAGE: separates proteins by size, and can be used to visualise proteins
- ELISA: uses antibodies to detect and quantify a specific protein in a sample### Enzyme Catalysis
- Enzymes are essential for biological reactions to occur, as they provide energy and overcome kinetic and thermodynamic barriers.
- The majority of enzymes are protein molecules, with some examples of RNA enzymes (ribozymes), such as the ribosome.
Thermodynamic Barriers
- A thermodynamic barrier refers to a reaction that is unfavourable in isolation and requires an input of energy to proceed.
- This can be overcome by an enzyme, which couples the reaction to a thermodynamically favourable reaction.
Kinetic Barriers
- A kinetic barrier refers to a reaction that does not proceed due to a temporary input of energy required to get over an energy barrier.
- Enzymes overcome kinetic barriers by stabilizing the transition state of a reaction, reducing the energy required to form the transition state molecule.
Properties of Enzymes
- Enzymes exhibit higher reaction rates, often 10^8 to 10^14-fold faster than the same reaction in the absence of an enzyme.
- Enzymes are highly specific, selecting the correct substrate molecule and reaction pathway to produce the final chemical product.
- Enzyme-catalysed reactions can be regulated by small molecules, allowing the cell to control the activity of an enzyme.
- Enzymes can couple reactions together, allowing unfavourable reactions to proceed.
Enzymes as Drug Targets
- Enzymes are ideal targets for drug development, as they can be used to treat diseases such as diabetes by controlling the activity of enzymes in metabolic pathways.
- Examples of successful drugs include HIV protease inhibitors and inhibitors of the influenza neuraminidase.
Energetics of Chemical Reactions
- To understand enzyme catalysis, it is essential to understand the energetics of chemical reactions.
- Energetics involve the balance of enthalpy (heat) and entropy (disorder) changes in a reaction.
Entropy, Enthalpy, and Free Energy
- Enthalpy change (ΔH) is related to the release or uptake of heat in a reaction.
- Entropy change (ΔS) is a measure of the disorder of a system.
- Gibbs Free Energy (ΔG) relates entropy, enthalpy, and temperature (T) and determines whether a reaction can proceed.
Chemical Equilibrium and ΔG
- The free energy of a reaction is related to the final equilibrium position of a chemical reaction.
- The equilibrium position is defined by the ratio of the concentrations of reactants and products.
Standard Free Energies
- The standard free energy (ΔG°) of a reaction is the free energy for a reaction where the starting point is clearly defined and all chemical species are initially present at a concentration of 1M, pH 7, and temperature 298K (25°C).
- ΔG° is related to the equilibrium constant (K) by the equation ΔG° = -RTlnK.
Coupling Reactions
- If a reaction has a positive ΔG, it can be made to proceed by coupling it to a reaction with a negative ΔG.
- Biological systems use ATP as an energy store in reaction coupling.
Enzyme Mechanisms
- Enzymes couple chemical reactions to drive reactions forward.
- Enzymes use the energy released by hydrolysis of ATP to drive reactions.
- Enzymes reduce the energy of the transition state of a reaction, reducing the kinetic barrier and allowing the reaction to proceed.
The Active Site
- The active site of an enzyme is the location where the reaction takes place.
- The structure of the enzyme arranges the active site and places reactive amino acid side chains in precise positions to interact with and distort the substrate molecule.
- The active site is often a cleft in the surface of the enzyme and is complementary in shape to the transition state of the reaction.
The Active Site and Catalysis
- The active site is the location on the enzyme where the reaction takes place.
- The structure of the enzyme positions the active site and reactive amino acid side chains to interact with and distort the substrate molecule.
- The active site is often a cleft in the surface of the enzyme and is complementary in shape to the transition state of the reaction.
- An ideal active site is optimized for the three distinct steps of a catalytic reaction:
- Binding the substrate with sufficient affinity and specificity.
- Stabilizing the transition state.
- Allowing product release.
Strategies for Transition State Stabilization
- Enzymes use different strategies to stabilize the transition state, including:
- Distorting the substrate molecule to make it more reactive.
- Positioning basic groups to accept protons.
- Bringing reactants into close proximity.
- Preventing movement of reactive groups to prevent side reactions.
Case Studies
- Carbonic anhydrase:
- Catalyzes the interconversion of carbon dioxide and bicarbonate.
- Uses a zinc ion to distort the substrate water molecule.
- Positions a basic group to accept a proton.
- Brings the reactants into close proximity.
- Serine proteases:
- Use a reactive serine residue to attack the peptide bond.
- Have a catalytic triad of serine, histidine, and aspartic acid residues.
- Use an oxyanion hole to stabilize the transition state.
- Are highly specific for the substrate due to the shape of the active site.
- Asp proteases:
- Use two catalytic aspartic acid residues to stabilize the transition state.
- Use a water molecule to facilitate the reaction.
- Have a different mechanism than serine proteases.
Enzyme Kinetics and Control
- Enzyme kinetics is the study of the rate of an enzyme-catalyzed reaction.
- The Michaelis-Menten equation describes the relationship between the initial rate of reaction and the initial concentration of substrate.
- The Michaelis-Menten equation is:
- vo = (vMAX * [S]) / (KM + [S])
- The values of vMAX and KM describe different properties of the enzyme:
- vMAX is the maximum reaction rate possible.
- KM is the substrate concentration at which the rate of the reaction is half the maximal rate.
- Enzyme control is essential for cellular function and is often disrupted in diseases.
Classification of Enzymes
- Enzymes are classified into six main classes:
- Oxidoreductases: catalyze oxidation and reduction reactions.
- Transferases: catalyze the transfer of chemical groups.
- Hydrolases: catalyze hydrolytic cleavage reactions.
- Lyases: catalyze the cleavage of various chemical bonds.
- Isomerases: catalyze geometric changes.
- Ligases: catalyze the joining of molecules together.### Enzyme Kinetics and Inhibition
- Measuring enzyme activity: Measure the initial rate of reaction (v0) for different initial substrate concentrations ([S]) to determine vMAX and KM.
- Lineweaver-Burk plot: A plot of 1/v0 against 1/[S] that gives a straight line, allowing vMAX and KM to be determined from the gradient and intercept.
Enzyme Inhibitors
- Competitive inhibitors: Bind directly to the active site, competing with the substrate for access, reducing the rate of reaction. Examples: transition state analogues, HIV protease inhibitors.
- Non-competitive inhibitors: Don't affect substrate binding but prevent catalysis, reducing vMAX.
- Allosteric inhibitors: Alter the shape of the active site, affecting substrate binding and catalysis.
Irreversible Inhibition
- Covalent bond formation: Inhibitors form covalent bonds with a side chain in the active site, blocking substrate access.
- Examples: Nerve gases (DIPF, Sarin), suicide inhibitors (penicillin).
Reversible Covalent Modification
- Phosphate group addition/removal: Regulates enzyme activity through phosphorylation/dephosphorylation.
- Protein kinases: Add phosphate groups to Ser, Thr, or Tyr side chains.
- Phosphatases: Remove phosphate groups.
Zymogens and Irreversible Activation
- Synthesised in inactive form, activated by proteolytic cleavage.
- Examples: Digestive enzymes, blood clotting reactions.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.