Podcast
Questions and Answers
According to Dalton's Law, what determines the partial pressure of a gas in a mixture?
According to Dalton's Law, what determines the partial pressure of a gas in a mixture?
- The molecular weight of the gas.
- The gas's solubility in water.
- The percentage of that gas in the gas mixture. (correct)
- The gas's density relative to other gases.
If the total atmospheric pressure is 750 mmHg, and oxygen constitutes 20% of the air, what is the approximate partial pressure of oxygen?
If the total atmospheric pressure is 750 mmHg, and oxygen constitutes 20% of the air, what is the approximate partial pressure of oxygen?
- 375 mmHg
- 75 mmHg
- 550 mmHg
- 150 mmHg (correct)
How does altitude affect the concentration of atmospheric gases?
How does altitude affect the concentration of atmospheric gases?
- Altitude decreases the concentration of all atmospheric gases.
- Altitude has no effect on the concentration of atmospheric gases. (correct)
- Altitude increases the concentration of all atmospheric gases.
- Altitude decreases the concentration of only heavier gases like carbon dioxide.
What is the primary mechanism responsible for moving air in and out of the lungs during ventilation?
What is the primary mechanism responsible for moving air in and out of the lungs during ventilation?
According to the information provided, what are the partial pressures of oxygen and carbon dioxide in the alveoli, respectively?
According to the information provided, what are the partial pressures of oxygen and carbon dioxide in the alveoli, respectively?
Given a barometric pressure (PB) of 750 mmHg, a water vapor pressure (PH2O) of 47 mmHg, and a fraction of inspired oxygen (FiO2) of 0.5, what is the PAO2 if the PaCO2 is 40?
Given a barometric pressure (PB) of 750 mmHg, a water vapor pressure (PH2O) of 47 mmHg, and a fraction of inspired oxygen (FiO2) of 0.5, what is the PAO2 if the PaCO2 is 40?
Which of the following best describes Fick's Law of diffusion?
Which of the following best describes Fick's Law of diffusion?
According to Fick's Law, what change would decrease the rate of gas diffusion across the alveolar membrane?
According to Fick's Law, what change would decrease the rate of gas diffusion across the alveolar membrane?
According to Henry's Law, what factor directly influences the amount of oxygen that dissolves into the bloodstream?
According to Henry's Law, what factor directly influences the amount of oxygen that dissolves into the bloodstream?
Which statement best explains why carbon dioxide diffuses about 20 times faster than oxygen across the alveolar-capillary membrane?
Which statement best explains why carbon dioxide diffuses about 20 times faster than oxygen across the alveolar-capillary membrane?
Which condition would result in a decreased alveolar oxygen pressure (PAO2)?
Which condition would result in a decreased alveolar oxygen pressure (PAO2)?
Which of the following is considered a normal transit time for diffusion of oxygen and carbon dioxide across the alveolar-capillary membrane in a healthy adult at rest?
Which of the following is considered a normal transit time for diffusion of oxygen and carbon dioxide across the alveolar-capillary membrane in a healthy adult at rest?
Which condition primarily affects the 'A' component (Surface Area) of Fick's Law?
Which condition primarily affects the 'A' component (Surface Area) of Fick's Law?
What is the normal thickness of the alveolar capillary membrane?
What is the normal thickness of the alveolar capillary membrane?
Which of the following represents a scenario where gas exchange is perfusion limited?
Which of the following represents a scenario where gas exchange is perfusion limited?
How do pressure gradients influence the movement of gases in the lungs?
How do pressure gradients influence the movement of gases in the lungs?
Which of the following conditions increases alveolar tissue thickness, potentially affecting gas diffusion?
Which of the following conditions increases alveolar tissue thickness, potentially affecting gas diffusion?
What components are directly proportional to the diffusion rate based on Fick's Law?
What components are directly proportional to the diffusion rate based on Fick's Law?
If a patient's alveolar-capillary membrane thickens due to a disease, which component of Fick's Law is most directly affected?
If a patient's alveolar-capillary membrane thickens due to a disease, which component of Fick's Law is most directly affected?
A patient is at sea level with a $P_B$ of 760 mmHg, a $P_{H_2O}$ of 47 mmHg, and an $F_{IO_2}$ of 0.6. Their $P_{aCO_2}$ is 55 mmHg. What is their $P_{AO_2}$?
A patient is at sea level with a $P_B$ of 760 mmHg, a $P_{H_2O}$ of 47 mmHg, and an $F_{IO_2}$ of 0.6. Their $P_{aCO_2}$ is 55 mmHg. What is their $P_{AO_2}$?
According to Graham's Law, what is the relationship between the diffusion of a gas through a liquid and the solubility coefficient of the gas?
According to Graham's Law, what is the relationship between the diffusion of a gas through a liquid and the solubility coefficient of the gas?
According to Graham's Law, what is the relationship between the diffusion of a gas through a liquid and the square root of the weight of the gas?
According to Graham's Law, what is the relationship between the diffusion of a gas through a liquid and the square root of the weight of the gas?
Which of the following nine structures is NOT part of the alveolar-capillary membrane through which a gas molecule must diffuse?
Which of the following nine structures is NOT part of the alveolar-capillary membrane through which a gas molecule must diffuse?
What is the partial pressure ($P_{N_2}$) of nitrogen in the atmosphere at a total atmospheric pressure of 760 mmHg, given that nitrogen constitutes 78.08% of the air?
What is the partial pressure ($P_{N_2}$) of nitrogen in the atmosphere at a total atmospheric pressure of 760 mmHg, given that nitrogen constitutes 78.08% of the air?
What is the partial pressure of water vapor at a body temperature of 37°C?
What is the partial pressure of water vapor at a body temperature of 37°C?
According to Fick's Law, how does increasing the surface area (A) of the alveolar membrane affect the rate of gas diffusion?
According to Fick's Law, how does increasing the surface area (A) of the alveolar membrane affect the rate of gas diffusion?
How would you characterise the diffusion of carbon monoxide?
How would you characterise the diffusion of carbon monoxide?
Flashcards
Dalton's Law
Dalton's Law
Total pressure of a gas mixture equals the sum of the partial pressures of each gas.
Atmospheric Gases
Atmospheric Gases
Earth's atmosphere is composed mostly of nitrogen (N2), oxygen (O2), and trace gases.
Partial Pressure Calculation
Partial Pressure Calculation
Partial pressure is calculated by multiplying the barometric pressure by the gas percentage.
Altitude Effect on Gas Concentration
Altitude Effect on Gas Concentration
Signup and view all the flashcards
Pressure Gradient
Pressure Gradient
Signup and view all the flashcards
Gas Diffusion
Gas Diffusion
Signup and view all the flashcards
Alveolar Gas Equation
Alveolar Gas Equation
Signup and view all the flashcards
Fick's Law
Fick's Law
Signup and view all the flashcards
Alveolar-Capillary Membrane Layers
Alveolar-Capillary Membrane Layers
Signup and view all the flashcards
Henry's Law
Henry's Law
Signup and view all the flashcards
Graham's Law
Graham's Law
Signup and view all the flashcards
Perfusion Limited
Perfusion Limited
Signup and view all the flashcards
Diffusion Limited
Diffusion Limited
Signup and view all the flashcards
Study Notes
Dalton's Law
- Dalton's Law states that the total pressure exerted by a gas mixture equals the sum of the pressures exerted independently by each gas in the mixture.
- The pressure exerted by each gas (its partial pressure) is directly proportional to the percentage of that gas in the gas mixture.
Atmospheric Gases
- Earth's atmospheric gases include nitrogen (N2), oxygen (O2), carbon dioxide (CO2), and trace gases like argon.
- Partial pressure of each gas is determined by multiplying the barometric pressure (PB) by the percentage of gas in the atmosphere.
- At sea level with a normal barometric pressure of 760 mmHg, the partial pressure of oxygen is approximately 159.6 mmHg, calculated as: Atmospheric P O2 = 0.21 × 760.
- The total atmospheric pressure is the collective sum of partial pressures of all atmospheric gases: P N2, P O2, and P C O2, and trace gases.
- As altitude increases, atmospheric pressure decreases, which causes the partial pressure exerted by each gas to decrease.
- The concentration of all atmospheric gases remains the same at both high and low elevations.
- The percentage of oxygen remains approximately 21 percent both at sea level and at the top of Mt. Everest (253 mmHg = Pb).
Pressure and Diffusion Gradients
- A pressure gradient involves the movement of gas from an area of high pressure/concentration to an area of low pressure/concentration.
- Pressure gradients are the primary mechanism responsible for moving air in and out of the lungs during ventilation.
- Each individual gas (e.g., N2, O2, CO2, trace gases) moves in the same direction, either in or out of the lungs.
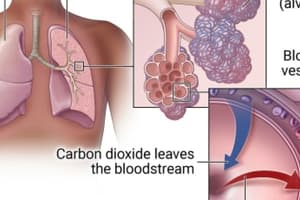
- Gas diffusion involves the movement of individual gas molecules independently from an area of high pressure/concentration to an area of low pressure/concentration.
Partial Pressure of Gases
- Partial pressures of gases vary in dry air, alveolar gas, arterial blood, and venous blood.
- P O2 levels: Dry air at 159.0, alveolar gas at 100.0, arterial blood at 95.0, and venous blood at 40.0.
- P C O2 levels: Dry air at 0.2, alveolar gas at 40.0, arterial blood at 40.0, and venous blood at 46.0.
- PH2O (water vapor): 47.0 across alveolar gas, arterial blood, and venous blood.
- PN2 (and other gases in minute quantities): 573.0 across alveolar gas, arterial blood, and venous blood.
- Total pressure in the air and alveoli is 760.0, 755.0 in the Arterial Blood and 706.0 in Venous Blood.
- Water vapor pressure varies based on temperature, with 47.0 mm Hg at 37°C when absolute humidity is 44.0 mg/L.
Ideal Alveolar Gas Equation
- Alveolar oxygen tension (PAO2) can be computed using the ideal alveolar gas equation: PAO2 = [PB – PH2O] FIO2 – PaCO2 / 0.8
Fick's Law
- Fick's Law explains how gas diffusion happens and is expressed as: V gas = A.D.(P₁ - P₂)/ T
- The layers an oxygen molecule crosses in the alveolar capillary membrane are: liquid lining, alveolar epithelial cell, basement membrane of the alveolar epithelial cell, interstitial space, basement membrane of the capillary endothelium, capillary endothelium, plasma of the capillary blood, erythrocyte membrane, and intracellular fluid in the erythrocyte.
- The area (A) component of Fick's Law is verified when alveolar collapse or alveolar fluid decreases the ability of oxygen to enter pulmonary capillary blood.
- 'D' denotes the diffusion constant, determined by Henry and Graham's Law.
- P1 - P2 represents the difference in partial pressure of the gas between two points.
- Decreased alveolar oxygen pressure (PAO2 or P1) caused by high altitude or alveolar hypoventilation reduces diffusion of oxygen into pulmonary capillary blood.
- The thickness (T) factor is confirmed when increased alveolar tissue thickness caused by alveolar fibrosis or alveolar edema reduces movement of oxygen across alveolar-capillary membranes.
- The normal thickness of the alveolar capillary membrane is 0.36-2.5 microns.
- The normal pressure gradient for oxygen across the alveolar-capillary membrane is 60 mm Hg when breathing room air at sea level, and the normal carbon dioxide gradient is 6 mm Hg.
Henry's Law
- Henry's Law explains how gases dissolve in the alveoli and bloodstream during gas exchange.
- The amount of oxygen that dissolves into the bloodstream is directly proportional to the partial pressure of oxygen in alveolar air.
- Oxygen has a high tendency to dissolve into deoxygenated blood because the partial pressure of oxygen is higher in alveolar air than in deoxygenated blood.
- Carbon dioxide diffuses out of the solution and back into gaseous form, having a greater partial pressure in deoxygenated blood than in the alveolar air.
Graham's Law
- Graham's Law says that the diffusion of a gas through a liquid is directly proportional to the solubility coefficient and indirectly proportional to the square root of the weight of the gas.
- Carbon dioxide diffuses about 20 times faster than oxygen because C02 is highly soluble in water, and co2 has a faster diffusion rate
- The solubility of CO2 is 24 times more than oxygen regarding the AC membrane.
- The weight of oxygen is lighter than Co2.
Diffusion and Perfusion Limitation
- Perfusion-limited gas transfer across the alveolar wall depends on the amount of blood that flows past the alveoli.
- Diffusion-limited gas movement across the alveolar wall depends on the integrity of the alveolar-capillary membrane itself.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.