Podcast
Questions and Answers
Flashcards
Gas Exchange
Gas Exchange
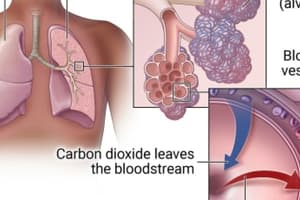
Gas exchange occurs between blood and alveolar air, across the blood-air barrier, depending on partial pressures of involved gases.
Dalton's Law
Dalton's Law
The sum of all the individual partial pressures of gases equals the total pressure exerted by the gas.
Henry's Law
Henry's Law
Henry's Law describes the solubility of a gas in relation to blood, where the amount of gas in solution is proportional to its partial pressure at a constant temperature.
Edema
Edema
Signup and view all the flashcards
Hypoxia
Hypoxia
Signup and view all the flashcards
Rate of Diffusion
Rate of Diffusion
Signup and view all the flashcards
Oxyhemoglobin Formation
Oxyhemoglobin Formation
Signup and view all the flashcards
Hypoxia
Hypoxia
Signup and view all the flashcards
Particle Pressures
Particle Pressures
Signup and view all the flashcards
Study Notes
- Partial pressure is described by Dalton's law.
Gas Exchange
- Gas exchange occurs between blood and the alveolar air.
- It happens across the blood-air barrier.
- The exchange depends on partial pressures of gases, not the concentration gradient.
- O2, CO2, and N have differing pressures.
- Diffusion occurs between gas and liquid, involving blood across the cell membrane.
Partial Pressures
- Described by Dalton's law.
- The sum of individual partial pressures equals the total pressure exerted by a gas.
- Ptotal = P1 + P2 + P3 + Pn...
- Air consists of ~21% oxygen, 79% nitrogen, and 0.03% carbon dioxide.
- At sea level: Ptotal = 760mm Hg (1 atm).
- Undersea: Ptotal = 2280mm Hg (3 atm).
- At altitude: Ptotal = 253mm Hg (0.33 atm).
- ATM changes percentages remain the same. While total and partial pressures, and concentration gradient, change.
- Each gas contributes to total pressure relative to its abundance.
- 760mmHg = O2(160mmHg) + N(600mmHg) + CO2(0.23mmHg) at SEA LEVEL.
- 2280mmHg = O2(479mmHg) + N(1801mmHg) + CO2(0.68mmHg) UNDERWATER.
- 2280mmHg = O2(479mmHg) + N(1801mmHg) + CO2(0.68mmHg) ALTITUDE.
- If the ATM pressure changes, gas percentages remain constant (21%, 79%, 0.23%).
- The total pressure changes (760, 2280, 253), and partial pressures change accordingly.
- Concentration gradient also changes.
Gas Exchange Into Liquid
- Diffusion between liquids and gases occurs according to Henry’s law.
- Henry’s law dictates a gas's solubility, and by extension, its diffusion, in relation to blood.
- C = k * P (concentration = solubility constant * pressure).
- As pressure increases, concentration increases (divers).
- As pressure decreases, concentration decreases (climb).
- At a constant temperature (k), the amount of gas in solution (C) is proportional to the partial pressure of that gas (P).
- Increased O2 at the lungs = Increased partial pressure.
- Decreased O2 at tissues = Decreased partial pressure.
- Important for the O2/CO2 method of travel.
- Henry's Law: C = k * P.
- When gas under pressure contacts a liquid (plasma), pressure forces gas molecules into solution.
- CO2 is more soluble than O2; nitrogen is more soluble.
- Underwater, at equilibrium, gas molecules diffuse out of the liquid as quickly as they enter it: number of gas molecules in solution is constant.
- O2/CO2 need to be transported in "liquid."
- Different gases will have different solubilities in liquid.
Where/Why is Gas Moving?
- PO2 is calculated based on free, dissolved O2 in plasma, not on the quantity of O2 bound to hemoglobin.
- Partial pressures is about what is in free solution, not bound to RBC.
- Amount of gas in solution (C) relates to partial pressure of that gas (P).
- High partial pressure = High concentration.
- Low partial pressure = Low concentration.
EDEMA
- Increased capillary permeability.
- This may be caused by an inflammatory response or infection.
- Increased fluid movement into the interdigital area.
- Protein also leaks into the interstitial compartment.
- Causes increased osmotic pressure in ISF.
- The body needs to apply pressure to stop the movement of proteins into the ISF.
- Holding additional fluid in the interstitial area.
Diffusion
- Diffusion problems can cause hypoxia.
- Diffusion rate is related to: surface area * concentration gradient * barrier permeability.
- Pathological changes might affect gas exchange.
- Decreased amount of alveolar surface area (all alveoli combined is less), caused by Emphysema.
- Increased thickness of alveolar membrane, caused by fibrotic lung diseases.
- Increased diffusion distance between alveoli and blood, caused by pulmonary edema.
Rate of Diffusion
The rate is affected by:
- Thickness of the membrane, which is impacted by edema (swelling)
- Surface area of the membrane, which is impacted by disease (Emphysema)
- Diffusion coefficient of gas. CO2 is highly soluble, O2 is somewhat less soluble, N has a low solubility at sea level.
- Partial pressure difference of gas between 2 sides of the membrane incoming O2 in the alveoli (40mmHg) vs. O2 in the conducting zone (100mmHg)
- Only one of the above factors can have an effect
- Exercise and altitude changes can increase demand, so RR/TV must increase the number of alveoli and tissues at equilibrium.
Gas Transport
- Gas transport described.
- Blood plasma alone cannot transport sufficient O2 or CO2, but red blood cells do.
- RBCs transport O2 and CO2 from peripheral tissues.
- They also remove O2 and CO2 from plasma, allowing gases to continue diffusing into the blood.
Oxygen
- O2 binds to iron ions in hemoglobin molecules in a reverse reaction.
- Creating oxyhemoglobin.
- Each red blood cell (RBC) has about 280 million Hb molecules.
- Each Hb molecule carries 4 oxygen molecules.
- Only 3.0 mL/100 mL arterial blood oxygen is dissolved in plasma (1.5%).
- The other 197 mL of arterial blood oxygen is transported by hemoglobin.
- Hb + O2 <=> Hb*O2.
- Hb = deoxyhemoglobin.
- The oxygenated blood travels to tissues, while deoxygenated blood travels to the lungs.
O2 Flow Summary
- Alveoli to plasma
- Membrane/ISF/membrane
- Plasma to RBC/Hgb
- RBC is from plasma to systemic tissues and then to plasma.
- Due to concentration gradients.
- Alveoli to plasma concentration is high.
- Plasma concentration is now higher than RBC, so it moves into RBC.
- At the same time, plasma concentration is reduced to move down its gradient into the plasma.
- Then, RBC to plasma.
- RBC concentration is higher, while plasma concentration is lower.
- Plasma concentration is higher than ISF.
- ISF concentration is higher than the cell/tissue concentration.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.