Podcast
Questions and Answers
What is the atomic number of Aluminum (Al)?
What is the atomic number of Aluminum (Al)?
13
What is the mass number of Aluminum (Al)?
What is the mass number of Aluminum (Al)?
27
In the equation $2PeO + \frac{1}{2}O_2 \rightarrow _$, what is the product formed?
In the equation $2PeO + \frac{1}{2}O_2 \rightarrow _$, what is the product formed?
2PeO_3
What is the atomic weight of Sodium (Na)?
What is the atomic weight of Sodium (Na)?
Signup and view all the answers
How many molecules are there in 2.2 NaQH according to the provided formula?
How many molecules are there in 2.2 NaQH according to the provided formula?
Signup and view all the answers
What is the method used to calculate the number of molecules?
What is the method used to calculate the number of molecules?
Signup and view all the answers
Study Notes
Chemical Equations
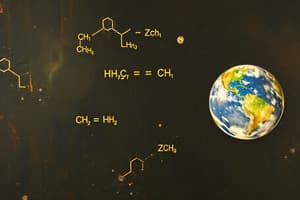
- The image shows handwritten calculations related to chemistry, particularly balancing equations and calculating the number of molecules.
Atomic Structure
- Aluminum (Al) has an atomic number of 13 and a mass number of 27.
- Aluminum has 13 protons and 14 neutrons.
Balancing Chemical Equations
- The equation $2PeO + \frac{1}{2}O_2 \rightarrow 2PeO_3$ demonstrates balancing a chemical reaction.
- This equation involves the reaction between $PeO$ and $O_2$ to produce $PeO_3$.
Molecular Calculations
- The equation $O_6 \ L_3 + 2.2 NaQH$ calculates the number of molecules in $2.2 NaQH$.
- The calculation utilizes atomic weights: Na = 23, O = 16, H = 1.
- The aim is to determine the number of moles and then convert this to the number of molecules.
- The equation used for this conversion is: $\frac{no.\ of\ moles}{20}=(no.of \ molecules)\times6.023\times 10^{23}$.
Notes
- Some of the symbols and chemical formulas in the image might not be perfectly standard. They appear like shorthand representations for better legibility.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.
Description
This quiz focuses on the balancing of chemical equations and molecular calculations, including atomic structure and the properties of aluminum. Test your understanding of how to balance reactions and calculate the number of molecules from given equations.