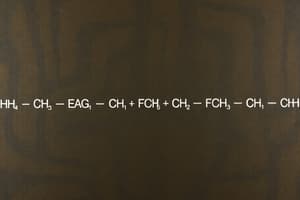Podcast
Questions and Answers
What is cheminformatics primarily focused on?
What is cheminformatics primarily focused on?
- Studying the physical properties of chemical compounds
- Developing computer hardware for chemical laboratories
- Facilitating the collection, storage, analysis, and manipulation of large quantities of chemical data (correct)
- Creating new chemical elements
What does a molecular graph in cheminformatics represent?
What does a molecular graph in cheminformatics represent?
- The color of a molecule
- The taste of a molecule
- The topology of a molecule (correct)
- The physical state of a molecule
How are acyclic molecules represented in cheminformatics?
How are acyclic molecules represented in cheminformatics?
- Using complex mathematical equations
- Using trees (correct)
- Using molecular graphs
- Using subgraphs
What is the main purpose of using generic, or Markush, structures in chemical disclosures in patents?
What is the main purpose of using generic, or Markush, structures in chemical disclosures in patents?
What does 'substituent variation' refer to in Markush structures?
What does 'substituent variation' refer to in Markush structures?
What is the purpose of 'fragment-screening' in the search system for Markush structures?
What is the purpose of 'fragment-screening' in the search system for Markush structures?
What does the 'reduced graph representation' in the search system involve?
What does the 'reduced graph representation' in the search system involve?
In the context of Markush structures, what does 'homology variation' refer to?
In the context of Markush structures, what does 'homology variation' refer to?
What is the purpose of the modified Ullmann algorithm in the search system for Markush structures?
What is the purpose of the modified Ullmann algorithm in the search system for Markush structures?
What method uses a hashing procedure to set bits to '1' in the fingerprint bitstring for any type of molecular structure?
What method uses a hashing procedure to set bits to '1' in the fingerprint bitstring for any type of molecular structure?
What is the role of patent databases in the context of chemical information retrieval?
What is the role of patent databases in the context of chemical information retrieval?
What is the purpose of the Smallest Set of Smallest Rings (SSSR) in chemical structure searching?
What is the purpose of the Smallest Set of Smallest Rings (SSSR) in chemical structure searching?
What is the purpose of the Morgan algorithm in chemical graph representation?
What is the purpose of the Morgan algorithm in chemical graph representation?
How is geometrical isomerism about double bonds indicated in SMILES notation?
How is geometrical isomerism about double bonds indicated in SMILES notation?
What is the purpose of hash keys in structure searching?
What is the purpose of hash keys in structure searching?
Flashcards are hidden until you start studying
Study Notes
Chemical Graph Representation and Structure Searching
- Isomorphic graphs have the same number of nodes and edges, and can be mapped to each other with equivalent nodes and edges.
- Connection tables communicate molecular graphs to and from computers, consisting of atomic numbers and bond pairs.
- Linear notation uses alphanumeric characters to encode molecular structures in a compact form.
- SMILES notation is a widely accepted linear notation, representing atoms by their atomic symbol and using upper and lower case for aliphatic and aromatic atoms.
- Geometrical isomerism about double bonds is indicated using slashes in SMILES notation.
- Canonical representation of molecular structures is crucial for avoiding duplicates in chemical database systems.
- The Morgan algorithm is a widely used method for determining a canonical order of atoms in a molecular graph, incorporating stereochemistry information.
- The CANGEN algorithm generates a unique SMILES string for each molecule using similar principles to the Morgan algorithm.
- Other formats for general utility include the canonical InChI representation by IUPAC and the Chemical Markup Language (CML).
- Structure searching converts the structure into a representation, allowing direct retrieval of information from the database through the generation of a hash key.
- Hash keys are integers used to retrieve information by moving the disk read mechanism directly to the location associated with the structure.
- Substructure searching identifies all molecules in the database containing a specified substructure, such as a particular functional group.
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.