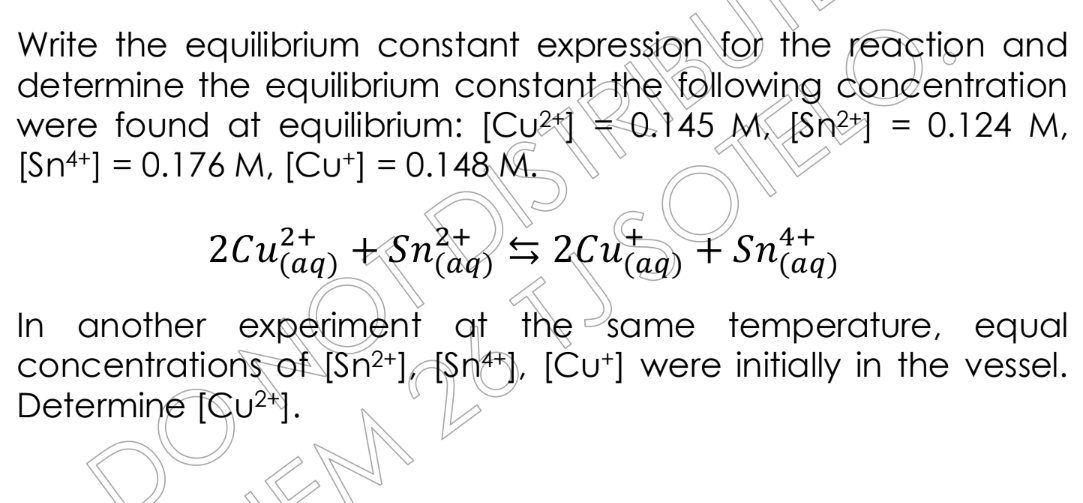
Write the equilibrium constant expression for the reaction and determine the equilibrium constant, given the following equilibrium concentrations: [Cu2+] = 0.145 M, [Sn2+] = 0.124... Write the equilibrium constant expression for the reaction and determine the equilibrium constant, given the following equilibrium concentrations: [Cu2+] = 0.145 M, [Sn2+] = 0.124 M, [Sn4+] = 0.176 M, [Cu+] = 0.148 M. The reaction is: 2Cu2+(aq) + Sn2+(aq) <=> 2Cu+(aq) + Sn4+(aq). In another experiment at the same temperature, equal concentrations of [Sn2+], [Sn4+], [Cu+] were initially present. Determine [Cu2+].
Understand the Problem
The question involves writing the equilibrium constant expression for a given chemical reaction and determining the equilibrium constant using provided concentrations. Additionally, it asks to calculate the concentration of Cu2+ in a separate experiment where initial concentrations of Sn2+, Sn4+, and Cu+ are equal.
Answer
K = ([Cu+]^2[Sn4+])/([Cu2+]^2[Sn2+]), K = 1.53, [Cu2+] = 0.070 M.
The equilibrium constant expression is K = ([Cu+]^2[Sn4+])/([Cu2+]^2[Sn2+]). The value of K is 1.53. In the second experiment, [Cu2+] = 0.070 M.
Answer for screen readers
The equilibrium constant expression is K = ([Cu+]^2[Sn4+])/([Cu2+]^2[Sn2+]). The value of K is 1.53. In the second experiment, [Cu2+] = 0.070 M.
More Information
The equilibrium constant, K, is a measure of the ratio of products to reactants at equilibrium. A K greater than 1 indicates that products are favored, while a K less than 1 indicates that reactants are favored.
Tips
A common mistake is to forget to raise the concentrations to the power of their stoichiometric coefficients when writing the equilibrium expression and calculating K.
Sources
- Calculating Equilibrium Constants - chem.purdue.edu
- 15.5: Calculating Equilibrium Constants - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information