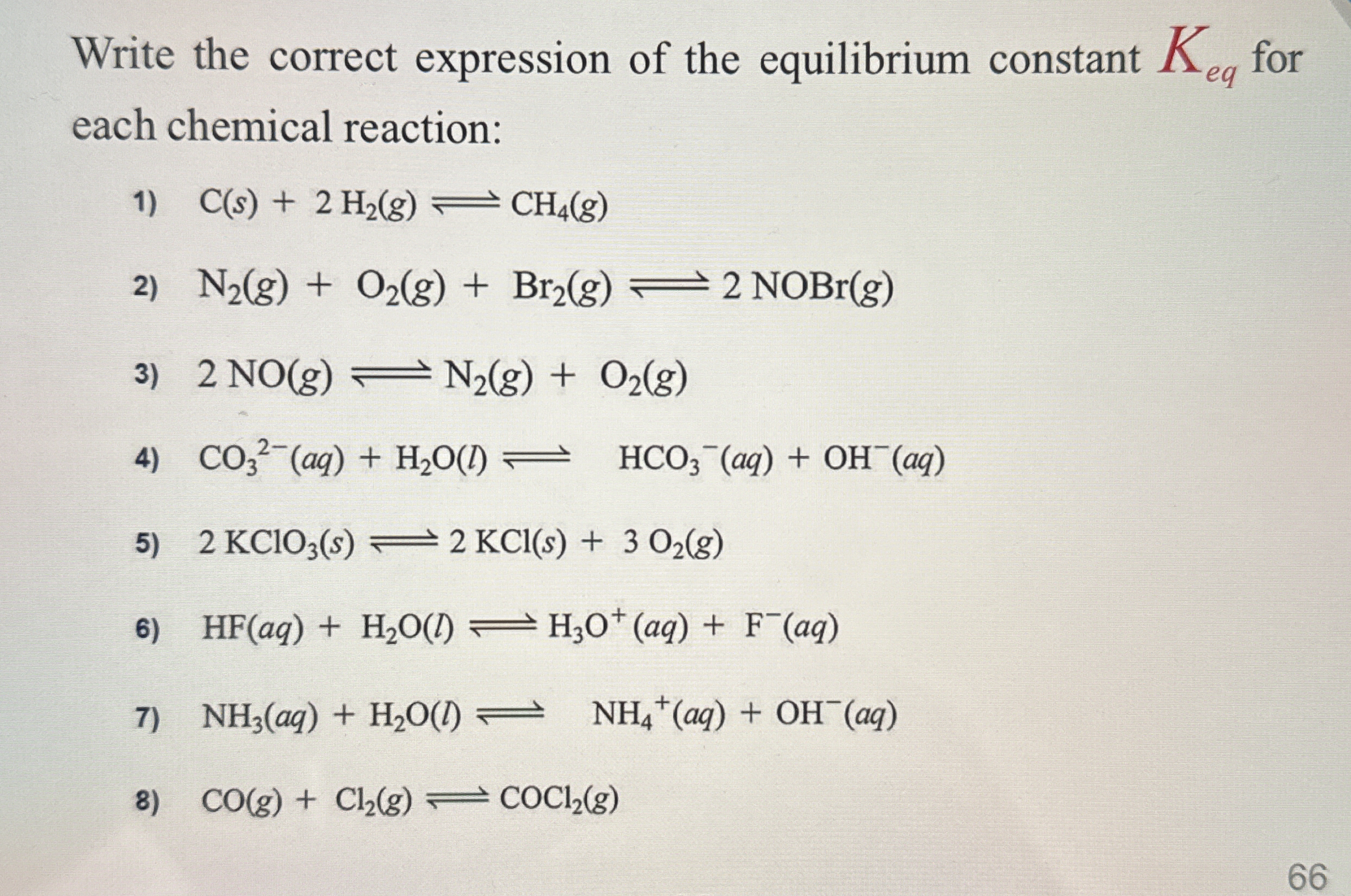
Write the correct expression of the equilibrium constant K_eq for each chemical reaction: 1) C(s) + 2 H2(g) ⇌ CH4(g) 2) N2(g) + O2(g) + Br2(g) ⇌ 2 NOBr(g) 3) 2 NO(g) ⇌ N2(g) + O2(g... Write the correct expression of the equilibrium constant K_eq for each chemical reaction: 1) C(s) + 2 H2(g) ⇌ CH4(g) 2) N2(g) + O2(g) + Br2(g) ⇌ 2 NOBr(g) 3) 2 NO(g) ⇌ N2(g) + O2(g) 4) CO3^2-(aq) + H2O(l) ⇌ HCO3^-(aq) + OH^-(aq) 5) 2 KClO3(s) ⇌ 2 KCl(s) + 3 O2(g) 6) HF(aq) + H2O(l) ⇌ H3O+(aq) + F^-(aq) 7) NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH^-(aq) 8) CO(g) + Cl2(g) ⇌ COCI2(g)
Understand the Problem
The question is asking to write the expression for the equilibrium constant (K_eq) for several chemical reactions presented. Each reaction involves different reactants and products, and the user needs to formulate the K_eq expressions accordingly.
Answer
1) [CH4]/[H2]^2 2) [NOBr]^2/([N2][O2][Br2]) 3) [N2][O2]/[NO]^2 4) [HCO3^-][OH^-]/[CO3^2-] 5) [O2]^3 6) [H3O^+][F^-]/[HF] 7) [NH4^+][OH^-]/[NH3] 8) [COCl2]/([CO][Cl2])
The equilibrium constant expressions are:
- K_eq = [CH4] / [H2]^2
- K_eq = [NOBr]^2 / ([N2][O2][Br2])
- K_eq = [N2][O2] / [NO]^2
- K_eq = [HCO3^-][OH^-] / [CO3^2-]
- K_eq = [O2]^3 (solids not included)
- K_eq = [H3O^+][F^-] / [HF]
- K_eq = [NH4^+][OH^-] / [NH3]
- K_eq = [COCl2] / ([CO][Cl2])
Answer for screen readers
The equilibrium constant expressions are:
- K_eq = [CH4] / [H2]^2
- K_eq = [NOBr]^2 / ([N2][O2][Br2])
- K_eq = [N2][O2] / [NO]^2
- K_eq = [HCO3^-][OH^-] / [CO3^2-]
- K_eq = [O2]^3 (solids not included)
- K_eq = [H3O^+][F^-] / [HF]
- K_eq = [NH4^+][OH^-] / [NH3]
- K_eq = [COCl2] / ([CO][Cl2])
More Information
Equilibrium constant expressions depend on the phase of the substances involved. Concentrations of gases and aqueous substances are included, while pure solids and liquids are not.
Tips
A common mistake is to include solids or liquids in the equilibrium expression. They should be omitted.
AI-generated content may contain errors. Please verify critical information