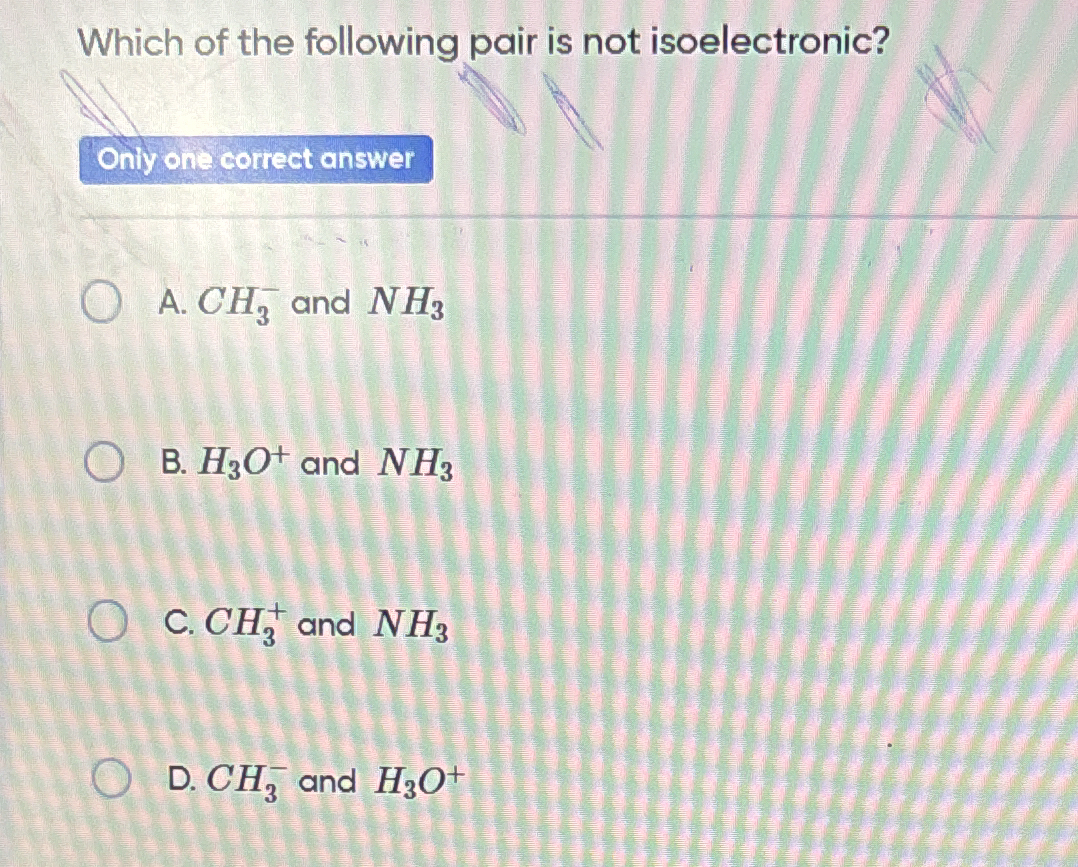
Which of the following pair is not isoelectronic?
Understand the Problem
The question is asking which of the listed pairs of chemical species are not isoelectronic, meaning they do not have the same number of electrons.
Answer
H3O+ and NH3 are not isoelectronic.
The pair H3O+ and NH3 is not isoelectronic.
Answer for screen readers
The pair H3O+ and NH3 is not isoelectronic.
More Information
Isoelectronic species have the same number of electrons. NH3 has 10 electrons whereas H3O+ has 9 electrons.
Tips
A common mistake is to forget to account for charges when counting electrons.
AI-generated content may contain errors. Please verify critical information