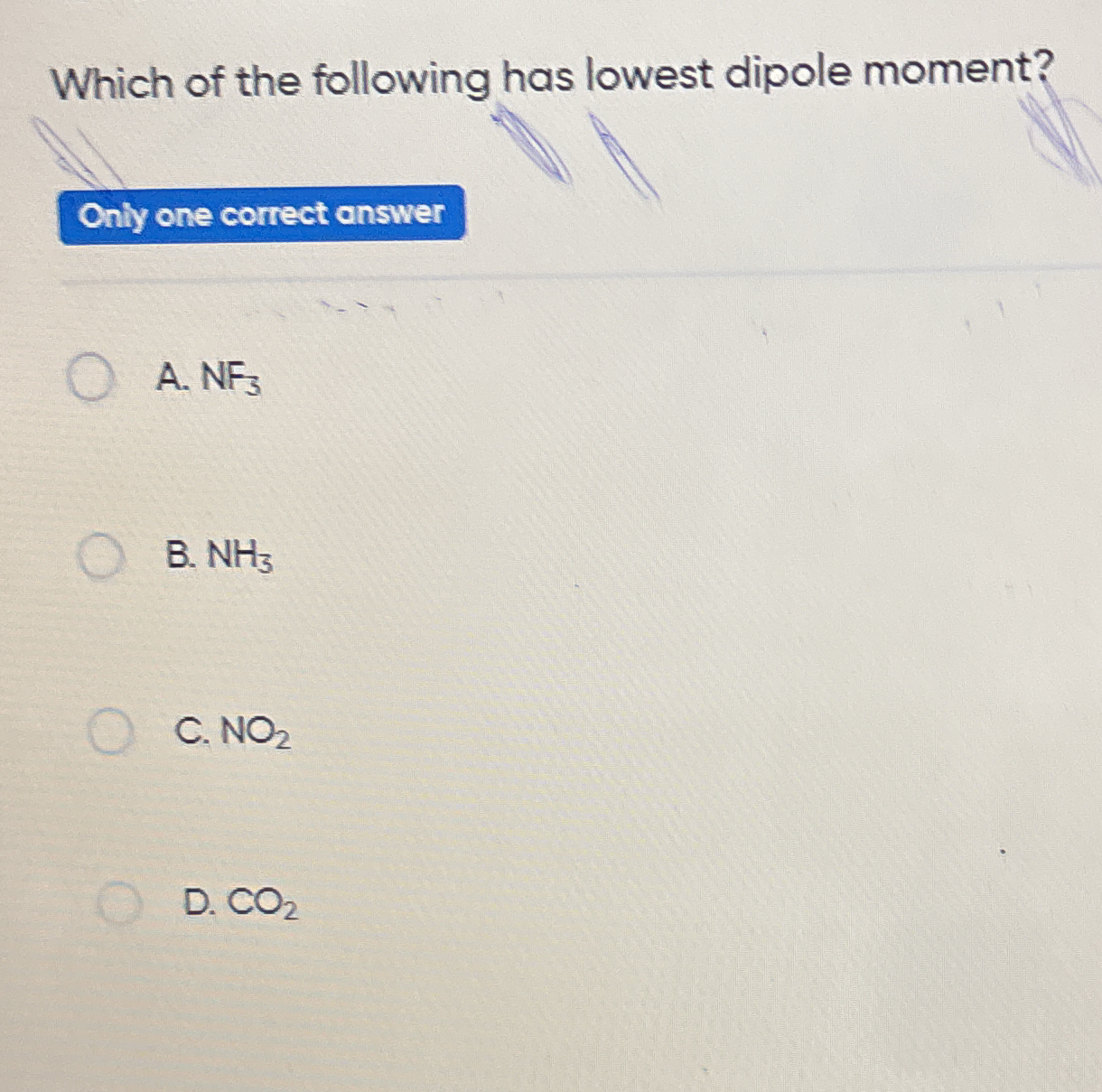
Which of the following has lowest dipole moment?
Understand the Problem
The question is asking which compound among the given options has the lowest dipole moment. We will analyze the molecular structures and electronegativities of the options provided to determine the answer.
Answer
CO2
The final answer is CO2, which has a dipole moment of zero due to its linear symmetry.
Answer for screen readers
The final answer is CO2, which has a dipole moment of zero due to its linear symmetry.
More Information
CO2, with its linear shape, has opposing dipoles canceling each other out, resulting in a zero dipole moment. This is unlike NF3, NH3, and NO2, all of which have inherent asymmetry causing non-zero dipole moments.
Tips
A common mistake is to assume that all molecules with polar bonds have dipole moments, but symmetry can lead to a net zero dipole moment.
Sources
- Molecular Dipole Moment Explained - chem.libretexts.org
- Chemistry: CO2 and Its Dipole Moment - toppr.com
AI-generated content may contain errors. Please verify critical information