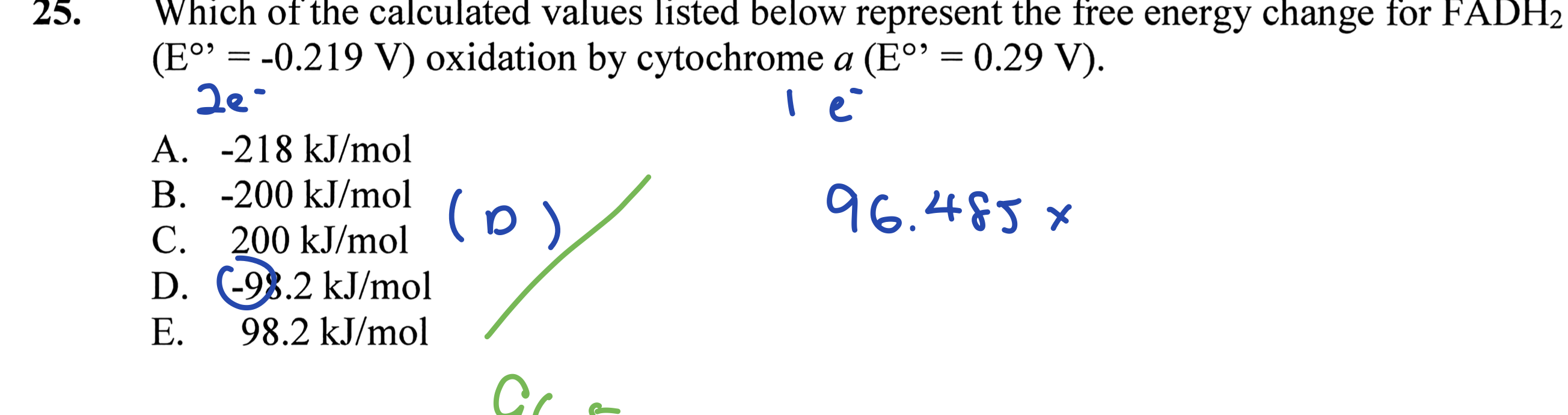
Which of the calculated values listed below represent the free energy change for FADH2 oxidation by cytochrome a?
Understand the Problem
The question asks which of the given calculated values represents the free energy change for the oxidation of FADH2 using the provided standard reduction potentials. The user needs to apply the Nernst equation or the relationship between Gibbs free energy and cell potential to determine the correct answer.
Answer
-98.2 kJ/mol
The final answer is -98.2 kJ/mol.
Answer for screen readers
The final answer is -98.2 kJ/mol.
More Information
The calculation uses the formula ΔG° = -nFΔE°', reflecting the relationship between free energy change and cell potential in redox reactions.
Tips
Make sure to use the correct values for the cell potentials and the Faraday constant. Watch for sign errors when calculating ΔE°'.
AI-generated content may contain errors. Please verify critical information